FRONTLINE FOCUS
A Relentless Focus on Safety
We are proud to share inspiring and exciting quality improvements at Children’s Wisconsin in this year’s Diagnostic, Anesthesia and Surgical Health Annual Report. As the leaders of the surgical program at Children’s Wisconsin, we also want to recognize our efforts to improve the health of children who come to us for surgical care through our focus on safety in a high-risk environment.
This focus arose from deep reflection on feedback we received in our annual Children’s Wisconsin provider and staff survey. More than 80% of providers and staff across the system completed our 2023 annual survey on engagement, inclusion and safety, giving us high scores compared to peer organizations. In fact, we earned the Gallup Exceptional Workplace Award based on our participation and scores. There was so much to be proud of, and it would’ve been easy to be content. Though the survey responses suggested that providers and staff feel we provide very safe care and work well as a team, they also highlighted some concerns.
Our leadership team wanted to learn more, so we started meeting with providers and staff to learn more and listen to their thoughts and concerns. We heard great stories about the work our teams do to keep our patients safe, and the ways in which our current workflows prioritize safety practices. However, team members also shared that not everyone feels empowered to speak up in the moment. Sometimes, people are afraid to share feedback for fear of adverse consequences. We also heard that we sometimes don’t follow through on suggested changes and solutions. Some of this feedback was hard to hear and pointed to the need for meaningful change.
To start, we re-committed to a safety program built on three pillars:
1. Communication: We will have the courage to speak up for safety and welcome feedback with grace and gratitude. We will approach difficult decisions with curiosity and assume positive intent.
2. Process: We will use data both to guide decisions and to review, develop and improve systems.
3. Accountability: We will hold ourselves and others accountable in all we do. We will acknowledge how our role contributes to patient safety and will own our part to address opportunities for improvement without blame, retaliation or shame.
To bring all team members together to share our commitment, we implemented a safety stand-down. On two mornings in late January 2024, we paused all elective cases at the Milwaukee Hospital and Surgicenter to bring our teams together to talk openly about safety. Though delaying cases is never an easy decision, we felt that this instance sent a message to our teams that we value how they feel at work. We value it so much that we “stopped the line” to talk about it together.
Since then, we’ve taken meaningful steps toward our goals. We kicked off a safety coach program and held our first safety council meeting in July. We began gathering data for a new safety dashboard to share with all our teams. We’re hearing from the teams that our culture is starting to reflect our shared commitment to transparency and safety. Most importantly, when something doesn’t feel right, more people feel safe speaking up and being heard.
We are committed to ongoing improvement. While there is still work to be done, the foundation of our safety culture is stronger than ever.
Patient Drug Exposure in Anesthesia
Many factors determine which medications anesthesiologists administer
Given the principle of “first do no harm,” one may posit that less is more in anesthesiology. However, recent changes in anesthesia strategies include using more agents to target the range of endpoints in the balanced anesthesia framework while reducing side effects and complications from any single agent.
Postoperative side effect
Many parents and providers are not aware that the most predictable effect of surgery and anesthesia in children is the behavioral alteration in the days, weeks or months following surgery. The biopsychological paradigm is post-traumatic stress disorder, which occurs in more than 50% of children in some studies. The most effective preventive strategy for postoperative behavior disturbance is the preoperative administration of a benzodiazepine.
At Children’s Wisconsin, we have a nearly universal strategy to administer preoperative midazolam to reduce postoperative behavioral disturbance. In the past two decades, our preoperative nurses have administered more than 250,000 doses. With serious complications in fewer than 1 in 10,000 doses, we have an amazing record of safety and effectiveness.
Defining the anesthetic state
Safe induction of the anesthetic state depends on the application of a range of medications to achieve defined, patient-specific endpoints. Experience-related outcomes are difficult to assess in children, who do not have access to continuous consciousness before age 2 and cannot reliably report what they have experienced until much later. However, we do have a structural foundation for the anesthetic state, commonly dissected into four distinct components:
1. Hypnosis (sleep), the unconscious state, which is distinct from but closely related to amnesia
2. Analgesia, which includes reduction in both nociception (pain receptor activation and afferent signaling in the peripheral and central nervous system) and its conscious perception
3. Immobilization, which includes muscle relaxation to facilitate procedural care without patient movement
4. Autonomic control, including direct and indirect modulation of neurohumoral responses to a hostile environment and support of organ system function by supplementation or replacement of neurohumoral agents as indicated
Every agent used to induce the anesthetic state has patient- and state-specific effects, and recognition of the patterns of responses is the major task of years of clinical training and experience for pediatric anesthesiologists.
Moving to balanced anesthesia
The potential for adverse effects of anesthetic drug exposure was highlighted in a U.S. Food and Drug Administration (FDA) alert and drove the creation of SmartTots, a public-private partnership between the FDA and the International Anesthesia Research Society, to support further research. Over the past decade, inquiry into the basic mechanism and possible toxicities of anesthetic agents has focused on cellular molecular mechanisms, integrative neurophysiology, analysis of complex neural pathways during the anesthetic state and later biobehavioral and developmental outcomes. Cellular and animal models show that both volatile anesthetic agents (sevoflurane, isoflurane, desflurane, halothane and methoxyflurane) and injectable hypnotic agents (benzodiazepines, propofol, ketamine and barbiturates) produce measurable cellular and ultrastructural effects with longer, higher-dose exposures. While some of these cellular effects are concerning, others provide protection against hypoxic-ischemic and inflammatory injury, thereby mitigating some of the deleterious effects of surgical trauma itself.
One way to reduce exposure to a single anesthetic agent is the concept of balanced anesthesia, in which multiple classes of drugs are administered to target separate endpoints and reduce the dose of any specific agent. The major classes of non-hypnotic agents in balanced anesthesia include opioids, neuromuscular blockers and anti-arousal autonomic suppressants (i.e., liberal local infiltration and major regional, spinal and epidural administration of local anesthetics). Each class of drugs has specific considerations for effect and side effects, and poly-drug approaches introduce complexity and the potential for deleterious interaction. Still, most patients now receive a balanced anesthetic approach with exposure to a range of medications. Most importantly, recent epidemiologic evidence shows that neurodevelopment is not impaired by general anesthesia, compared to spinal anesthesia in infants,1 or by general anesthesia, compared to no anesthesia in hospitalized infants and children.2 Recent evidence is reassuring about both short-term safety and long-term outcomes related to anesthesia in infants and children.3
Medication data
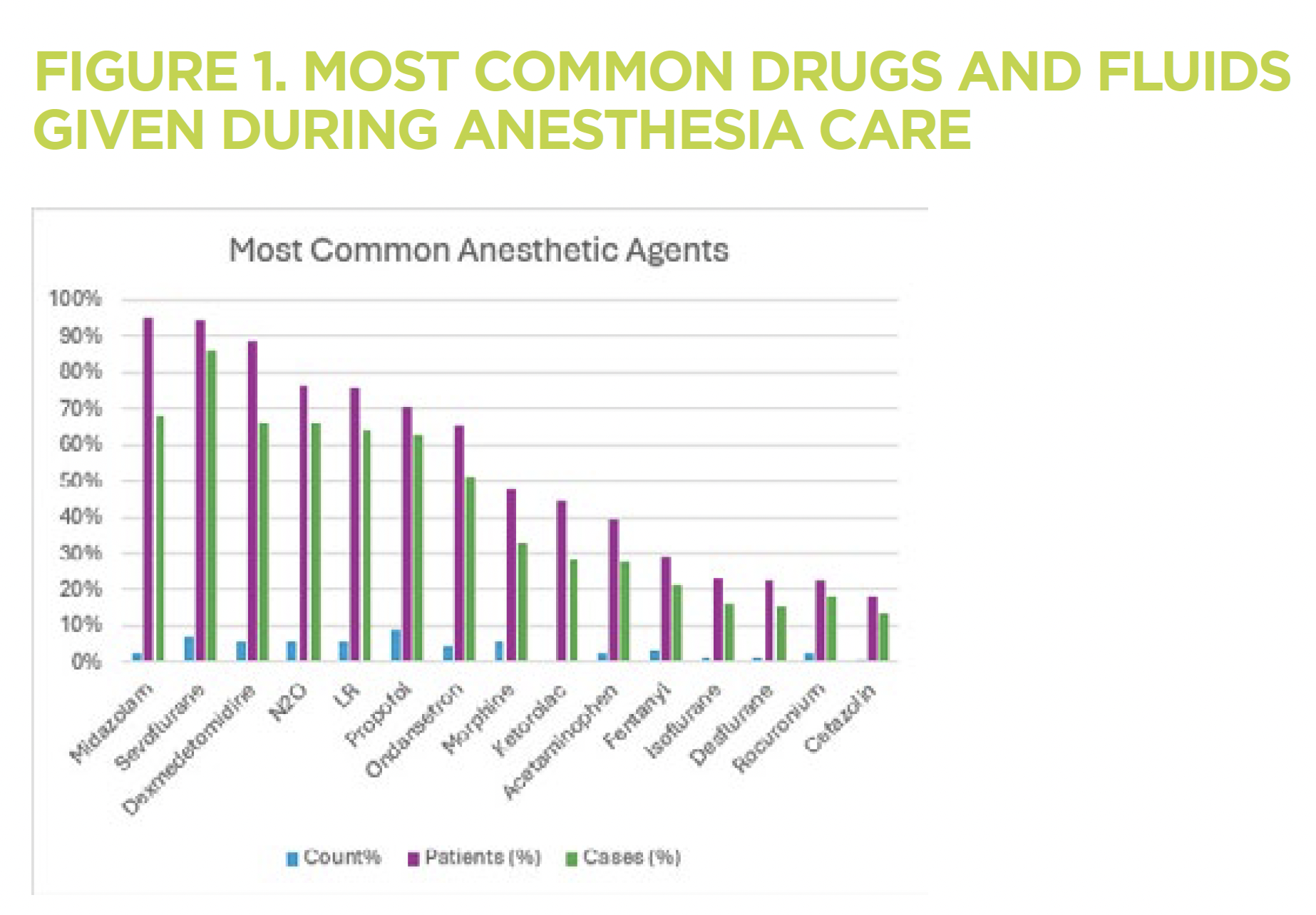
We have cataloged all anesthetic drug administration at Children’s Wisconsin since 2013. In 275,208 cases, 3,299,914 administrations of drugs occurred in 142,298 patients. The most common agents are shown in Figure 1.
The pattern of drug administration has changed, influenced by emerging evidence and technologic development, allowing precision drug administration, facilitating the more widespread application of balanced anesthesia approaches. Comparing five pre-pandemic years (2013-2018) to a recent period (2020-2024), we see a shift toward shorter-acting, multimodal anesthesia strategies, including more analgesic, anti-emetic agents (acetaminophen, dexmedetomidine, ketorolac) and local anesthetics (Figure 2). The use of anticoagulants (heparin) and vasoactive drugs (phenylephrine) also increased.
At Children’s Wisconsin, we are dedicated to following the best available scientific evidence, combined with practical and humane considerations, to provide individualized, safe and patient-centered anesthesia care for every patient.
George M. Hoffman, MD
Anesthesiologist-in-Chief, Children’s Wisconsin Chief and Professor of Anesthesiology,
Medical College of Wisconsin
References
1. McCann ME, Berde C, Soriano S, Marmor J, Bellinger D, de Graaff JC, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet, Feb 16 2019;393:664–77. doi:10.1016/S0140-6736(18)32485-1.
2. Silber JH, Rosenbaum PR, Reiter JG, Jain S, Hill AS, Hashemi S, et al. Exposure to Operative Anesthesia in Childhood and Subsequent Neurobehavioral Diagnoses: A Natural Experiment using Appendectomy. Anesthesiology, Sept 1 2024;141(3):489-499. doi:10.1097/ALN.0000000000005075.
3. Vutskits L, Culley DJ. GAS, PANDA, and MASK: No Evidence of Clinical Anesthetic Neurotoxicity! Anesthesiology, Oct 2019;131:762-4. doi:10.1097/ALN.0000000000002863.
Preventing Respiratory Adverse Events
Understanding respiratory drive may improve perioperative anesthesia care
In children, respiratory adverse events are the most frequently encountered adverse events in the perioperative period. Many events are due to upper airway obstruction and increased airway reactivity, especially in patients with underlying lung disease, asthma or adenotonsillar hypertrophy. Respiratory tract infections increase the incidence of adverse events and cannot always be avoided. In addition to airway and lung function, anesthetics and opioids also significantly affect the central nervous mechanisms of respiratory control.
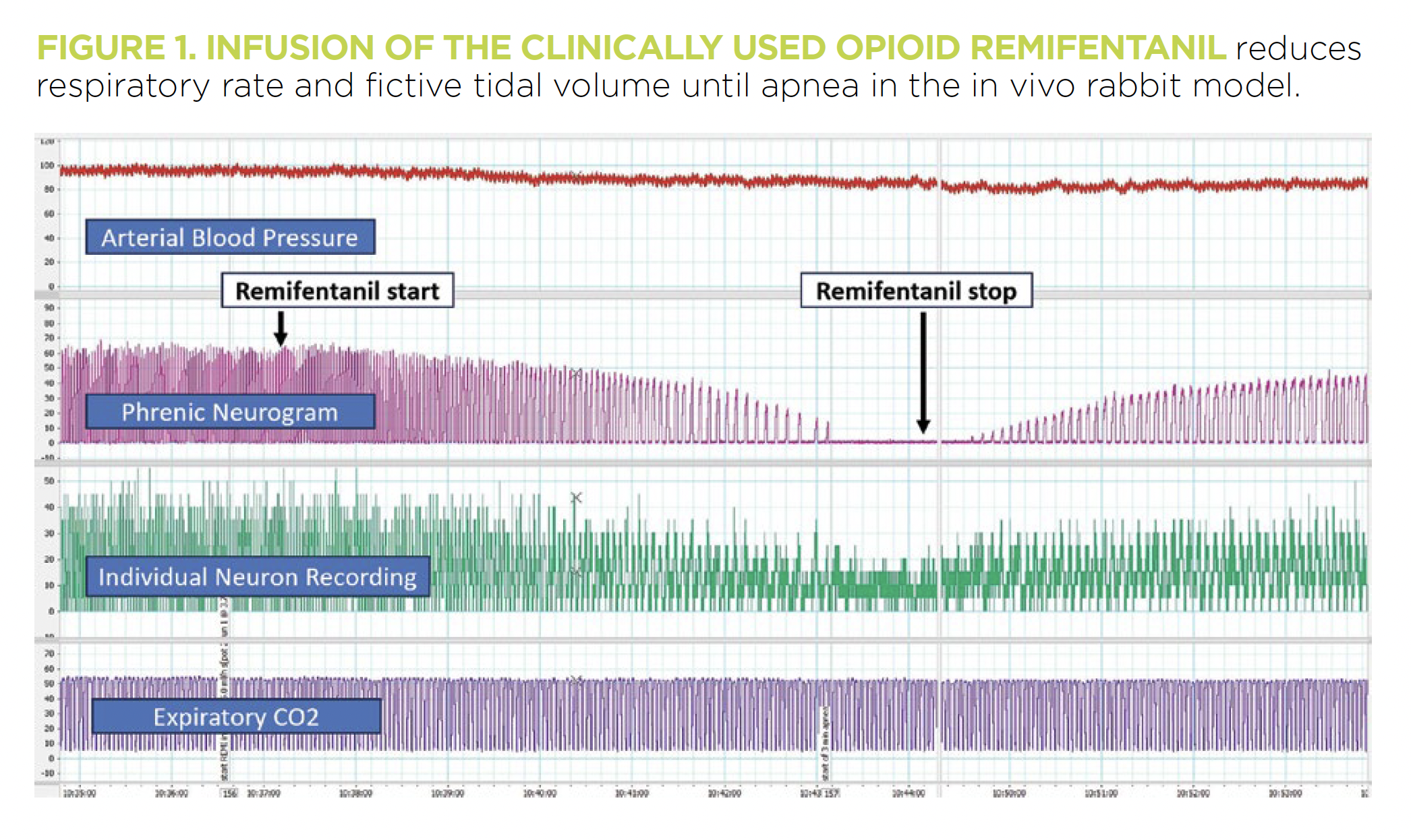
As an anesthesiologist responsible for her patients’ breathing in the perioperative period, I was motivated to investigate a mechanistic understanding of respiratory physiology in a basic science model. Based on many years of central respiratory control research by Edward Zuperku, PhD, and Eckehard Stuth, MD, I developed a new, decerebrate in vivo rabbit model with a developmental aspect. This animal model retains most of the physiologic reflexes found in mammals and allows the investigation of multiple functionally identified brainstem sites and drug mechanisms without confounding baseline anesthesia (Figure 1).
The Stucke lab focuses on three aspects:
1. The neuronal mechanisms, particularly the specific neuron types regulating the duration of inspiration, expiration and tidal volume
2. How opioids and anesthetics depress breathing by affecting these neurons
3. How respiratory stimulating drugs increase the activity of these neurons
Effects of opioids
We are particularly interested in why the beneficial effect of all respiratory stimulating agents is limited at high opioid doses. Over time, we have made several important discoveries: “Breathing” does not solely depend on the preBötzinger complex, a neuronal network in the medulla that generates the basic respiratory rhythm. Respiratory phase duration also depends critically on inputs from the pontine respiratory group, while inputs from chemosensitive areas contribute to phase duration in the preBötzinger complex and directly affect tidal volume through their projections to premotor and motoneurons.
We showed that opioids depress breathing through direct effects on the preBötzinger complex and also, dose-dependently, on the pons, the medullary raphe and phrenic motoneurons. So far, we are the only lab to completely reverse the respiratory depressant effects of intravenous opioids with multiple consecutive microinjections of the antagonist naloxone into specific brainstem areas. This highlights the importance of these areas and provides a road map for further investigation.
Studying adverse events
With the support of the Diagnostic, Anesthesia and Surgical Health team at Children’s Wisconsin, we are working on two projects investigating perioperative respiratory adverse events and the effect of sleep on respiratory drive and phase timing. The former project, in collaboration with Richard Berens, MD, and George Hoffman, MD, uses data from the electronic health record to quantify the incidence of adverse respiratory events, postoperative escalation of care and other outcome variables. The latter is based on a theoretical model of respiratory drive derived from our animal studies that explains the exponential nature of respiratory drive on respiratory rate. This helps us understand why the same dose of an opioid or sedative can cause substantially more respiratory depression in patients who have received other respiratory depressant agents or whose respiratory drive is reduced at baseline, such as young infants.
Astrid G. Stucke, MD
Director of Research, Division of Pediatric Anesthesiology, Children’s Wisconsin Professor of Pediatric Anesthesiology, Medical College of Wisconsin
At Children’s Wisconsin, we perform an average of 50 intracranial cases annually through our multidisciplinary Head Shape Clinic.
At Children’s Wisconsin, we perform an average of 50 intracranial cases annually through our multidisciplinary Head Shape Clinic.
Enhanced Recovery after Cranial Vault Surgery
Optimizing postoperative outcomes through multimodal pain management and perioperative protocols
Craniosynostosis is the premature fusion of cranial sutures and occurs in 1 in 2,000 to 25,000 live births. Surgery is usually indicated in the first year of life to prevent intracranial hypertension and improve the appearance of the shape of the head. Optimizing perioperative and postoperative factors can improve the recovery process for these children. Adequate pain control is a priority in all pediatric surgical patients. In addition to enhancing patient comfort, effective pain management can improve recovery after surgery. Patients with craniosynostosis undergoing cranial vault remodeling (CVR) present a unique challenge as they are often preverbal and require the use of behavior-related pain scales for medication administration. Additionally, the use of analgesics may conceal signs of neurological complications.
We continuously evaluate our surgical process to delineate modifiable factors that could influence postoperative outcomes and pain management. At Children’s Wisconsin, we perform an average of 50 intracranial cases annually through our multidisciplinary Head Shape Clinic.
Reduced narcotic use
When looking at CVR, we found that increased perioperative narcotic use was associated with increased length of hospital stay, respiratory events and episodes of emesis. Therefore, current pain regimens should emphasize a multimodal approach to pain with limited narcotic use to enhance postoperative recovery. We then wanted to evaluate factors that would result in the administration of fewer narcotics but still maintain appropriate analgesia and patient comfort for the recovery process. When looking at perioperative factors, we found that increased fluid requirements intraoperatively, longer operative durations and placement of drains resulted in higher narcotic administration. Conversely, dexmedetomidine administration, as well as postoperative NSAIDs, are associated with decreased narcotic requirements.
Optimized perioperative care
Blood transfusion is a commonly evaluated factor for cranial vault outcomes. We found that children who received NSAIDs had a lower blood transfusion rate, indicating that they did not experience any clinically significant additional bleeding. The administration of intraoperative tranexamic acid was also associated with a decreased blood transfusion volume postoperatively.
Modifying perioperative factors such as these may lead to decreased blood transfusions and decreased postoperative pain, which may reduce patients’ narcotic requirements, improving their overall outcomes. We plan to create an enhanced recovery after surgery protocol to implement a standard perioperative regimen to improve outcomes for children following cranial vault surgery.
Kristen A. Klement, MD
Pediatric Plastic Surgeon, Children’s Wisconsin Associate Professor of Pediatric Plastic Surgery, Medical College of Wisconsin
Improving Outcomes Following Congenital Heart Surgery
Cell-free DNA helps identify the most vulnerable patients with congenital heart disease
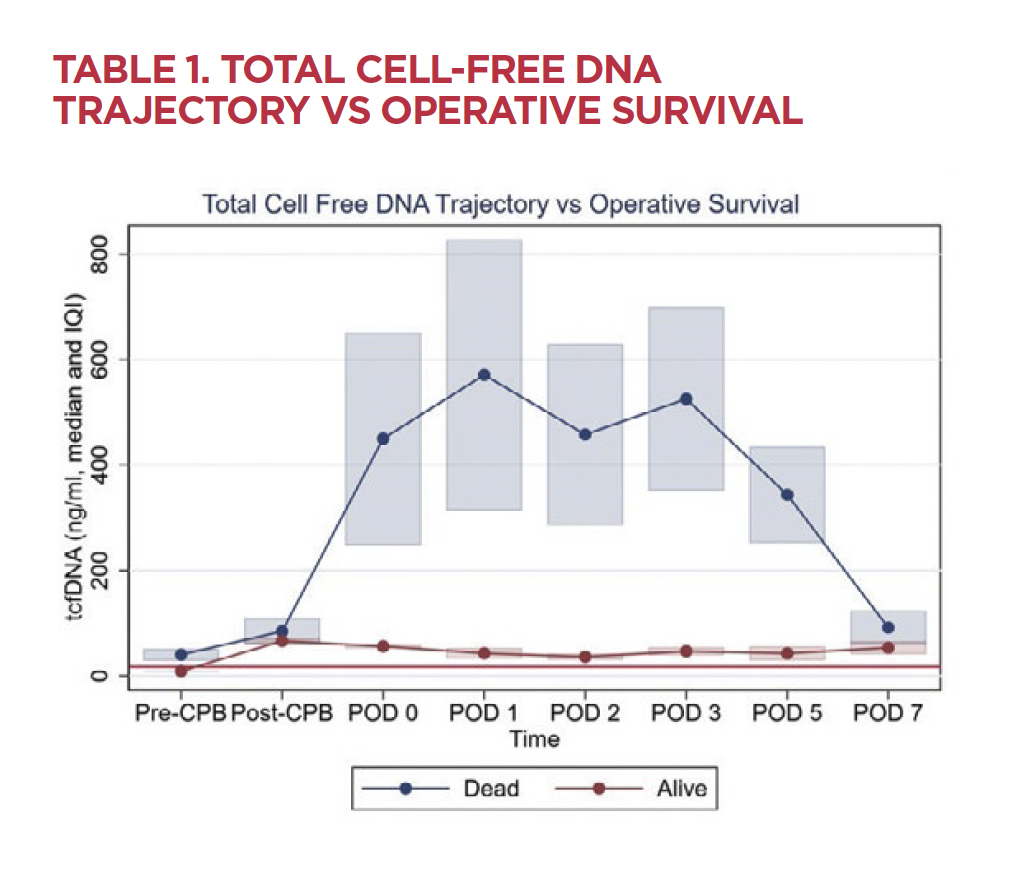
One percent of children are born with congenital heart disease (CHD), and 30% of these children require surgery during childhood. Despite advances in care, mortality rates remain greater than 3%, with major postoperative morbidity occurring in 20%. These statistics have stimulated the pursuit of methods for the early identification of vulnerable patients to allow for intervention.1 These methods include predictive scoring systems, laboratory biomarkers and advanced noninvasive oximetric monitoring.
What we’ve learned
Cell-free DNA (cfDNA) is a biomarker of nuclear and mitochondrial origin that is produced at low levels during states of health and cleared rapidly from circulation. Various disease states cause elevation of cfDNA, including, but not limited to, sepsis, trauma and infection.
The cardiothoracic team at Children’s Wisconsin has focused on leveraging the power of cfDNA to improve outcomes in children with CHD. We initially studied cfDNA in children who had received heart transplants and discovered that elevated donor-specific cfDNA predicted rejection risk, and elevated total cfDNA predicted risk of death and need to treat for infection.2 These findings are significant for heart transplant recipients who must undergo repeated interventional procedures and cardiac biopsies to evaluate for rejection. The possibility of a blood test obviating the need for these procedures would be a dramatic improvement in the care of these patients.
We then turned our attention to children who require open-heart surgery for CHD. Our group performed a prospective observational study in 116 children undergoing cardiac surgery at Children’s Wisconsin and found that the level of nuclear cfDNA predicted a composite outcome of cardiac arrest, the need for extracorporeal membrane oxygenation (ECMO) or mortality.3 Unlike other predictive models, this study identified a preoperative marker with the potential to predict which children are likely to need postoperative care escalations.
Analysis from this study also compared nuclear cfDNA with mitochondrial cfDNA. Cardiac myocytes are rich in mitochondria due to these cells’ energy demands, which makes mitochondrial cfDNA specifically a very attractive biomarker. Our group found that a combined analysis of both nuclear and mitochondrial cfDNA postoperatively can enhance the risk assessment of these patients for mortality and hospital length of stay.4
Current studies
Our group is currently performing a prospective observational study in neonates and infants undergoing cardiac surgery and comparing cfDNA to cardiac troponin, another biomarker of cardiac injury. We are focusing on children less than one year of age due to their increased vulnerability perioperatively. We aim to further refine the predictive ability of this biomarker and soon bring the bench to the bedside to identify at-risk patients and intervene earlier for the most vulnerable.
Extracellular DNA serves as a pro-inflammatory mediator, and cfDNA may not only serve as a biomarker for disease severity but also as a therapeutic target to reduce perioperative inflammation. Our group is currently investigating whether the DNA cleavage enzyme dornase alpha, utilized as a nebulized formulation for individuals with cystic fibrosis, can reduce the concentration of cfDNA in an ex vivo model of ECMO. This work may serve as a springboard for future animal and clinical investigations to reduce inflammation from congenital heart surgery and improve outcomes.
Through our group’s work to investigate the predictive ability of cfDNA and develop risk stratification schema and increased enzymatic clearance, we continue to strive to meet the need for ongoing improvement in the care of children with CHD.
Jake P. Scott, MD
Medical Director and Chief, Pediatric Anesthesiology, Children’s Wisconsin
Professor of Anesthesiology and Pediatrics, Medical College of Wisconsin
Justinn M. Tanem, MD
Pediatric Anesthesiologist and Critical Care Specialist, Children’s Wisconsin
Assistant Professor of Pediatric Anesthesiology and Critical Care, Medical College of Wisconsin
Kevin Daley
Perfusion Supervisor, Children’s Wisconsin
Tracy R. Geoffrion, MD, MPH
Congenital Heart Surgeon, Children’s Wisconsin, Assistant Professor of Congenital Heart Surgery, Medical College of Wisconsin
George M. Hoffman, MD
James S. Tweddell Chair in Pediatric Cardiothoracic Research, Associate Medical Director (Emeritus) of Pediatric Cardiac Critical Care, Anesthesiologist-in-Chief, Children’s Wisconsin Professor of Anesthesiology and Pediatrics, Medical College of Wisconsin
Michael E. Mitchell, MD
Medical Director of Pediatric Cardiothoracic Surgery, Children’s Wisconsin
Chief and Professor of Pediatric Cardiothoracic Surgery, Medical College of Wisconsin
Aoy Tomita-Mitchell, PhD
Investigator, Children’s Research Institute, Professor of Surgery and Biomedical Engineering, Medical College of Wisconsin
References
1. Jacobs JP, He X, Mayer JE Jr, Austin EH 3rd, Quintessenza JA, et al. Mortality Trends in Pediatric and Congenital Heart Surgery: An Analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. Oct 2016;102(4):1345-52. doi: 10.1016/j.athoracsur.2016.01.071. Epub Aug 31 2016. PMID: 27590683.
2. Scott JP, Ragalie WS, Stamm KD, Mahnke DK, Liang HL, et al. Total Cell-Free DNA Predicts Death and Infection Following Pediatric and Adult Heart Transplantation. Ann Thorac Surg. Oct 2021;112(4):1282-1289. doi: 10.1016/j.athoracsur.2020.08.006. Epub 2020 Oct 8. PMID: 33039362.
3. Tanem JM, Scott JP, Hoffman GM, Niebler RA, Tomita-Mitchell A, et al. Nuclear Cell-Free DNA Predicts Adverse Events After Pediatric Cardiothoracic Surgery. Ann Thorac Surg. Aug 2023;116(2):349-356. doi: 10.1016/j.athoracsur. 2022.10.027. Epub Nov 1 2022. PMID: 36332680.
4. Scott JP, Tanem JM, Tomita-Mitchell A, Hoffman GM, Niebler RA, Liang HL, Simpson PM, Stamm KD, North PE, Mitchell ME. Elevated nuclear and mitochondrial cell-free deoxyribonucleic acid measurements are associated with death after infant cardiac surgery. J Thorac Cardiovasc Surg. Aug 2022;164(2):367-375. doi: 10.1016/j.jtcvs.2021.10.066. Epub Dec 24 2021. PMID: 35144816.
At Children’s Wisconsin, our Cardiac and Pediatric ICU teams collaborate closely with surgical colleagues through co-rounding, ensuring exceptional care for every patient.
At Children’s Wisconsin, our Cardiac and Pediatric ICU teams collaborate closely with surgical colleagues through co-rounding, ensuring exceptional care for every patient.
Quality Through Collaboration
Working together improves care for our critically ill surgical patients
In 2023, there were 845 surgical admissions to the Cardiac Intensive Care Unit (CICU) and Pediatric Intensive Care Unit (PICU) at Children’s Wisconsin. We work closely with our surgical colleagues through co-rounding to achieve improved patient care for these patients. At interdisciplinary rounds, we focus on minimizing risk from hospital-acquired conditions (HACs) by reviewing the indication for lines/tubes that are present, as well as emphasizing initiation of early enteral nutrition and ensuring agreement on the care plan. This collaboration aids with seamless transitions to the acute care floor when critical care services are no longer needed. Joint development of clinical practice guidelines — such as those on traumatic brain injury and the Norwood postoperative clinical pathway — also facilitates our patients’ best and safest care.
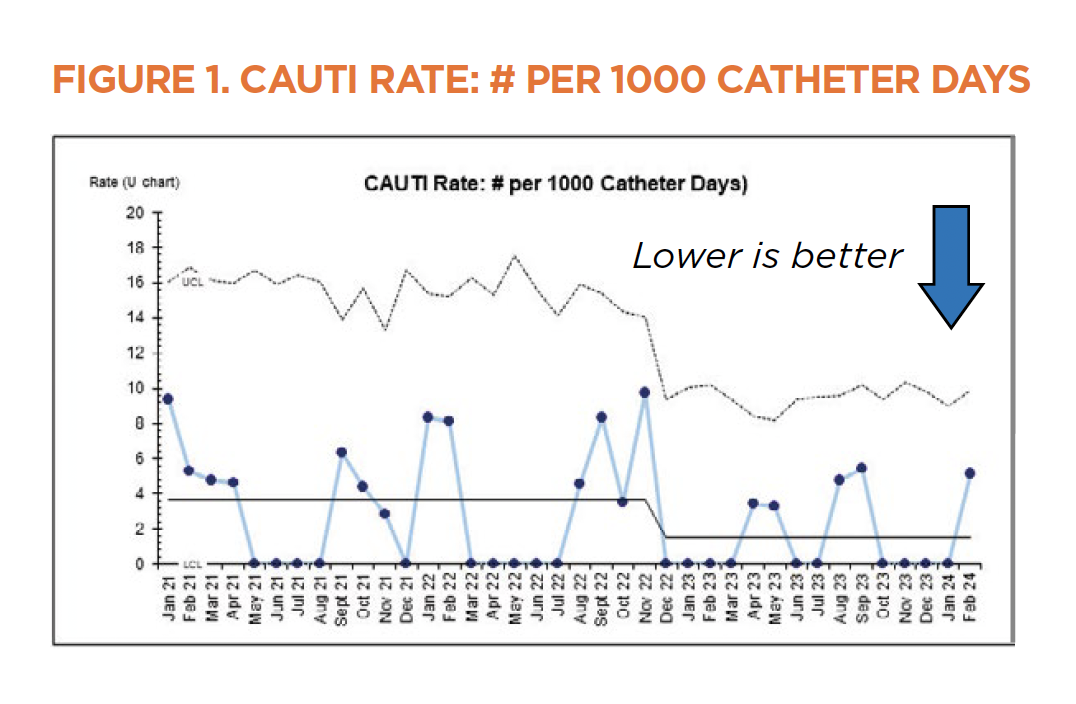
HAC risk reduction
The patients in the CICU and PICU — especially those who are postoperative — are among the patients at highest risk for developing HACs due to factors such as critical illness, impaired mobility and the presence of lines/tubes. Close partnerships with our surgical and nursing colleagues have led to improvements in the rates of unplanned extubations, catheter-associated urinary tract infections (CAUTI) and hospital-acquired pressure injuries (HAPI) in our patients. The control chart for the CAUTI rates over the past few years (Figure 1) shows a shift in the center line, indicating the overall CAUTI rate is meaningfully lower.
The CAUTI, HAPI and central line-associated bloodstream infection (CLABSI) teams review all events, identifying system improvement opportunities. The CLABSI and CAUTI teams also regularly round on patients with central lines and Foley catheters to review indications and clarify policies and procedures.
The HAPI team recently focused on reducing pressure injuries from electroencephalogram (EEG) leads, which led to a 31% decrease in EEG lead-related pressure injuries from 2022 to 2023. The HAPI team is working on reducing pressure injuries from cervical collars, with close collaboration from nursing, trauma surgery, neurosurgery and neurocritical care teams.
Ongoing quality collaboratives
The critical care section is highly involved in several multi-institutional collaboratives. The CICU is a member of the Pediatric Cardiac Critical Care Consortium (PC4), which aims to provide outcome data for benchmarking to identify local quality improvement opportunities. Recent projects through PC4 have included evaluating cardiac surgery-associated acute kidney injury in neonates undergoing the Norwood operation, analyzing the care and outcomes of critically ill children with clinically diagnosed myocarditis and developing and implementing chylothorax clinical practice guidelines.1
The CICU and PICU are members of PediRES-Q, an international collaborative that aims to identify patients at high risk of cardiac arrest and improve intra-arrest and post-arrest care. Recent projects have included the roll-out of “situational awareness” signs at the bedside that identify why a patient is at high risk of cardiac arrest and the proposed mitigation plan for bedside staff, as well as the development of an in situ mock code curriculum. As part of this collaborative, we will be a site for investigating Physiology-directed Epinephrine Dosing in Cardiac Arrest (PEDICA), recently funded by the National Institutes of Health.
Lastly, the PICU is a member of PICU UP!, a collaborative focused on a bundled intervention that facilitates early mobility for our critically ill patients.2 Early mobility in critically ill adults has been shown to improve outcomes.3 This pediatric quality improvement initiative aims to show similar benefits in the duration of mechanical ventilation and length of stay.
We are dedicated to collaboration because it improves care for all our patients, including our critically ill surgical patients.
Jenny Andres, MBA, BSN, RN
Quality and Outcomes Manager, Children’s Wisconsin
Andrea R. Maxwell, MD
Critical Care Specialist, Children’s Wisconsin, Assistant Professor of Pediatric Critical Care, Medical College of Wisconsin
Martin K. Wakeham, MD
Pediatric Critical Care Specialist, Children’s Wisconsin Professor of Pediatric Critical Care, Medical College of Wisconsin
Matthew C. Scanlon, MD
Pediatric Critical Care Specialist, Children’s Wisconsin Professor of Pediatric Critical Care, Medical College of Wisconsin
References
1. Lion RP, Winder MM, Amirnovin R, et al. Development of consensus recommendations for the management of postoperative chylothorax in paediatric CHD. Cardiol Young. 2022;32(8):1202-1209. doi:10.1017/S1047951122001871.
2. Wieczorek B, Ascenzi J, Kim Y, et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children. Pediatr Crit Care Med. 2016;17(12):e559-e566. doi:10.1097/PCC.0000000000000983.
3. Sosnowski K, Lin F, Chaboyer W, Ranse K, Heffernan A, Mitchell M. The effect of the ABCDE/ABCDEF bundle on delirium, functional outcomes, and quality of life in critically ill patients: A systematic review and meta-analysis. Int J Nurs Stud. 2023;138:104410. doi:10.1016/j.ijnurstu.2022.104410.
The State of Oral Health
How we’re bridging gaps in pediatric oral health and advancing comprehensive care
Nearly 25 years ago, Surgeon General David Satcher first described the state of oral health in disadvantaged populations as a “silent epidemic.” Sadly, dental caries (a common chronic infectious disease resulting from tooth-adherent cariogenic bacteria) continues to be the most common childhood disease, and access to care is further out of reach for too many.
Roughly 40% of children experience caries by the time they start kindergarten. If left untreated, caries causes pain and infection. It limits a child’s ability to eat, speak and sleep, distracts them from learning and playing and negatively affects self-esteem.
Caries is more pervasive in children living in families with lower incomes, children with special health care needs (CSHCN) or children who have craniofacial differences, such as cleft lip and palate. These children are twice as likely to have caries and half as likely to visit the dentist.
Oral health matters
The oral health status of children in Wisconsin mirrors national trends, as about 1 in 3 children have untreated dental decay. More than half of the counties in Wisconsin have a shortage of dentists, and only 29% of dentists accept Medicaid. More than 85% of those we serve are Medicaid-insured.
Children’s Wisconsin has provided dental care since 1930. Because children travel from across the state for our expert care, we are now one of Wisconsin’s largest pediatric dental care providers, with more than 27,000 visits generated annually across four clinic locations. The CSHCN population represents about 35% of those served. Nearly 1,500 dental general anesthesia cases were completed in 2023.
Knowing that oral health is integral to overall health, we aligned our strategy to ensure that a child’s physical, mental, social and dental needs are met. We created synergy between our oral health, surgical, craniofacial and plastics service lines to integrate medical and dental care and implement joint program development to address the dental needs of all children. Here’s how this strategy worked for two of the kids we serve:
Case 1: Efficient, collaborative care
Addie was born with Down syndrome, and as she grew, other medical conditions were identified, including a severe brain bleed, right-sided hemiplegic cerebral palsy and autism. Addie’s mom had trouble finding a dental provider near their home who would care for Addie — not only because of Addie’s needs but because she had Medicaid insurance. When Addie had other procedures under anesthesia, like getting ear tubes, we coordinated with her medical team to include dental care as part of her procedure.
Case 2: Expert care with compassion
Lici was diagnosed with an ultra-rare neurodevelopmental disease associated with the CAMK2B gene. Lici experiences global delays, uses a wheelchair and is fed through a J-tube overnight. Due to her complex health needs, Lici visits our clinic for regular cleanings and preventive care. Lici’s mom shared that she trusts our team to be thorough and explain things clearly. If Lici needs dental work or has cavities in the future, she would likely need treatment under general anesthesia — a service we are uniquely equipped to provide.
Surgical health
Creating a partnership between oral health and surgical health was essential to manage the increasing number of children and CSHCN presenting with advanced caries. These children often require that care be completed under general anesthesia. With the stiff competition across specialties for operating room time, hospitals and surgery centers across the country significantly decreased or eliminated time for dental general anesthesia cases following the COVID-19 pandemic.
At Children’s Wisconsin, we found a way to allocate operating room time for our staff and community dentists. In doing so, we also maximized utilization and were afforded more flexibility to add on urgent cases. As the demand continues to outpace the time available, the team is exploring alternative approaches that are safe and effective and may reduce wait time, further maximize utilization and enhance the patient-family experience.
Pamela Fraser, MSM
Director, Oral Health Services, Children’s Wisconsin
References
1. Salerno, C, D’Avola, V, Oberti, L, Almonte, E, Bazzini, EM, Tartaglia, GM, Cagetti, MG. Rare Genetic Syndromes and Oral Anomalies: A Review of the Literature and Case Series with a New Classification Proposal. Children 2022, 9, 12. doi.org/10.3390/children9010012.
2. Guerreiro E, Botelho J, Machado V, Proença L, Mendes JJ, Manso AC. Caries Experience before and after COVID-19 Restrictions: An Observational Study. J Clin Med. Feb 19, 2024;13(4):1164. doi: 10.3390/jcm13041164. PMID: 38398476; PMCID: PMC10889374.
3. Amini H, Casamassimo PS, Litch CS, Fosse C, Boynton JR. Impact of Reduced Operating Room Access on Dental Departments in Children’s Hospitals. Pediatric Dent. Nov 15, 2023;45(6):504-509. PMID: 38129750.
4. Ritwik P, Khan FM. Evaluating Options and Ethics in Pediatric Dentistry due to Declining Access to Hospital Operating Rooms. J Clin Ethics. Summer 2023;34(2):211-217. doi: 10.1086/724228. PMID: 37229734.
5. Nilz M, Sharma D, Schneider S, Vithalani A. 165 Trends in Emergency Department Dental Pain Visits During the COVID-19 Pandemic. Ann Emerg Med. Oct 2022;80(4): S74–6. doi: 10.1016/j.annemergmed.2022.08.189. Epub 2022 Sep 29. PMCID: PMC9519225.
6. Capriola S. Wisconsin Healthy Smiles Survey—Kindergarten and Third Grade Children, 2024. Wisconsin Department of Health Services Oral Health Program. Publication number P-00589. dhs.wisconsin.gov/publications/p0/p00589.pdf.
About 1 in 3 children in Wisconsin have untreated dental decay. Children’s Wisconsin is working to ensure that their dental needs are met.
About 1 in 3 children in Wisconsin have untreated dental decay. Children’s Wisconsin is working to ensure that their dental needs are met.
Increasing Wellness for Children with Obesity
Lifestyle Medicine Collaborative is a new option for multidisciplinary care
Childhood obesity has been an ongoing concern in the United States for the last three decades.1 Recent data shows that the rate of children and adolescents affected by obesity is 1 in 5 (20%),1 and the rate of adult obesity is predicted to be around 50% by 2030.2
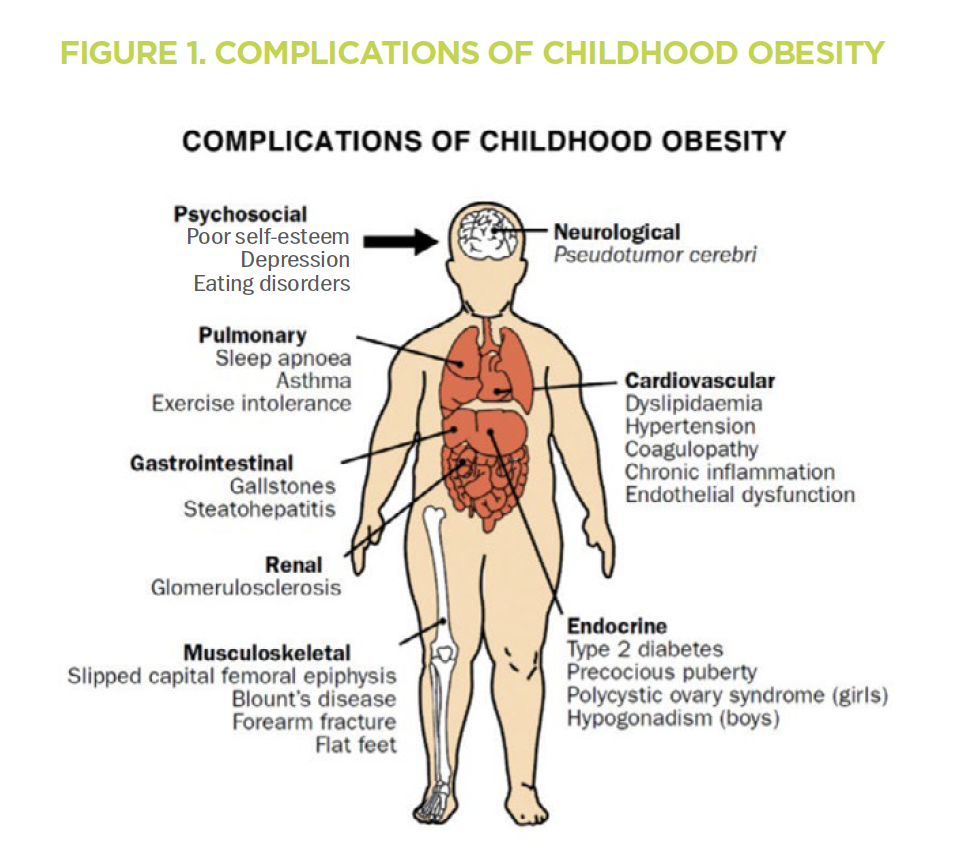
Overweight and obese status is defined based on body mass index (BMI) (Table 1).3 A child who is overweight or obese is at significant risk of developing co-morbid conditions involving multiple organ systems (Figure 1).6 These include metabolic dysfunction-associated steatotic liver disease (MASLD), type 2 diabetes mellitus, hyperlipidemia and hypertension. Up to 45% of children with overweight or obese status will suffer from at least one co-morbid condition,4 and many have multiple concurrent co-morbidities. Hence, the Lifestyle Medicine Collaborative at Children’s Wisconsin was created in 2023 to provide comprehensive care for children with multiple co-morbid conditions. It works collaboratively with other programs at Children’s Wisconsin to provide specialized care for overweight and obese children.
Options for care
Nutrition, Exercise and Weight Management (NEW) Kids Program has been a staple of the GI subspecialty clinics at Children’s Wisconsin for more than 20 years. This program focuses on dietary and exercise changes that promote a healthier lifestyle for kids who meet overweight/obese criteria and have one co-morbidity.
Lifestyle Medicine Collaborative (LMC) provides patient-centered, comprehensive care to children with obesity and two or more associated co-morbidities. In this clinic, hepatologists, cardiologists, endocrinologists and registered dietitians collaborate to determine the best approach(es) for successful lifestyle changes and weight loss. This may include using weight loss medications and/or bariatric surgery.
Adolescent Bariatric Surgery Program pairs our obesity specialists at Children’s Wisconsin with Tammy L. Kindel, MD, PhD, a bariatric surgeon and medical director of the Bariatric Surgery Program at Froedtert & the Medical College of Wisconsin. This is the only such program in the state accredited to care for adolescents under 18.
Research and quality improvement
We are currently involved in two ongoing quality improvement (QI) studies assessing our patients with overweight or obese status. Denise is spearheading the IRB-approved study on the health outcomes of children who have completed the NEW Kids Program in the past decade. Dr. Mack is the PI on a QI project entitled “Prevalence of co-morbid conditions in children of overweight or obesity status at Children’s Wisconsin.” This retrospective study involves about 80,000 children of overweight or obese status who were cared for at Children’s Wisconsin from 2020 to 2023. Characterization of the frequency and types of obesity-related co-morbidities in these patients will reflect the largest comprehensive account of the burden of these pediatric co-morbidities in the United States.
Future focus
The LMC team set goals in three areas:
Clinical: Enhance the multidisciplinary approach to care by adding physical therapists for exercise management and psychologists to address the social, psychological and behavioral components accompanying weight-based concerns.
Research: Set the stage for future treatment trials to address the impact of weight loss medications on co-morbid conditions.
Advocacy: Increase engagement at the national level and advocate for insurance coverage for nutritional counseling, weight loss medications and metabolic bariatric surgery.
Our ultimate goal is to optimize health and quality of life for children and families affected by obesity.
The Lifestyle Medicine Collaborative at Children’s Wisconsin was created in 2023 to provide comprehensive care for children with multiple co-morbid conditions.
The Lifestyle Medicine Collaborative at Children’s Wisconsin was created in 2023 to provide comprehensive care for children with multiple co-morbid conditions.
Denise M. Kilway, APNP
Pediatric Gastroenterology Nurse Practitioner, Children’s Wisconsin, Assistant Professor of Pediatrics, Medical College of Wisconsin
Cara L. Mack, MD
Medical Director of Enteral Feeding, Children’s Wisconsin, Chief and Professor of Pediatric Gastroenterology, Hepatology and Nutrition, Medical College of Wisconsin
References
1. Centers for Disease Control and Prevention (CDC) (2024). Childhood Obesity Facts. Retrieved from cdc.gov/obesity/childhood-obesity-facts/childhood-obesity-facts.html.
2. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. NEJM 2019;381: 440-2450.
3. Hampl SE, Hassink SG, Skinner AC, et al. American Academy of Pediatrics Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023;151(2):1-93.
4. Pedicelli S, Fintini D, Rava L et al. Prevalence of prediabetes in children and adolescents by class of obesity, Pediatr Obes 2022;17(7):e12900.
5. Kansra R, Lakkunarajah S, Jay MS. Childhood and adolescent obesity: A review. Frontiers of Pediatrics 2021; doi: 10.3389/fped.2020.581461.
6. Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002 Aug 10;360(9331):473-82. doi: 10.1016/S0140-6736(02)09678-2. PMID: 12241736.
A Team Approach to Liver and Biliary Surgical Challenges
The Complex Liver/Hepatobiliary Program provides multidisciplinary care for complex problems
Liver and biliary diseases are some of the most complex surgical problems we face as pediatric surgeons. To provide the best quality care for each child we see, it is imperative that we work as a multidisciplinary team that includes surgery, hepatology, radiology, anesthesiology, critical care and pathology.
At Children’s Wisconsin, we initiated the Complex Liver/Hepatobiliary Program, spearheaded by pediatric surgery, transplant surgery and hepatology, as a collaborative effort to ensure we provide the best and most innovative care for these children. Our core team members work together to review decision-making for individual patients, coordinate diagnostic and therapeutic efforts and consider novel approaches for unique situations. The program goals are to maintain our tradition of excellent patient care and build upon this tradition to lead the field in our region and nation.
A complex diagnosis
Biliary atresia (BA) is a diagnosis that typifies the complex nature of pediatric surgical liver and biliary disease. Neonates are affected by BA shortly after birth, demonstrating obstructed bile drainage from the liver due to incompletely formed bile ducts. Progressive liver injury results and can lead to fibrosis, liver failure and the need for liver transplantation. Timely recognition and management are critical. In some instances, the bile duct obstruction can evolve, worsening over the first month or two of life, and can lead to difficulty making the diagnosis.
Affected neonates are managed with surgical intervention involving removing the obstructed and scarred bile duct remnants and creating an intestinal conduit attached to the surface of the liver hilum to allow for drainage. This operation, known as the “Kasai procedure,” is named after Morio Kasai, MD, and his pioneering work in developing this approach during the mid-20th century.
Each infant’s version of BA anatomy differs. Therefore, intraoperative flexibility is paramount to success. Despite the need for flexibility, variation in surgical practice has been a problem that limits achieving the best possible outcomes in BA, and it is now accepted that focusing the care for these complex neonates in a limited number of centers is likely beneficial. For many infants affected by BA, liver transplantation is indicated when a diagnosis is late or if liver fibrosis progresses from inadequate bile drainage despite the timely application of the Kasai procedure.
Speeding up decision-making
The definitive diagnosis of BA is often made using a cholangiogram, which involves accessing the gallbladder (if one is present and identifiable) with a catheter and infusing contrast to evaluate the patency and anatomy of the bile ducts using fluoroscopy. Traditionally, this is done directly via an open surgical approach using an incision in the right upper quadrant of the abdomen. More recently, the field has moved toward a laparoscopic approach.
At Children’s Wisconsin, we are one of a limited number of centers capable of performing cholangiograms using a percutaneous needle-based approach in collaboration with our interventional radiologists. When successful, this approach adds valuable information to the diagnostic evaluation of these neonates without an operation. Simultaneous liver biopsy streamlines data gathering and allows our team to make informed decisions earlier in the patient’s disease course.
Path to optimizing success
Each neonate affected by BA presents a series of challenging clinical decisions:
• What is the best and least invasive approach to definitively making
the diagnosis?
• When is the optimal time to deploy surgical intervention?
• Which aspects of the Kasai procedure would optimize success?
• Is the patient better served by liver transplantation rather than the Kasai
procedure as their initial surgical management strategy?
We are now working through our Complex Liver/Hepatobiliary Program to establish our local standards for each of these questions. We are strengthening our collaborative relationships with other leading centers in BA around the nation and seeking opportunities to participate in multi-institutional research efforts to clarify best practices and test novel approaches.
Brian T. Craig, MD
Pediatric General and Thoracic Surgeon, Children’s Wisconsin, Assistant Professor of Pediatric General and Thoracic Surgery, Medical College of Wisconsin
Raj Prasad, MD
Medical Director of Liver Transplant, Children’s Wisconsin, Professor of Transplant Surgery, Medical College of Wisconsin
Every diagnosis starts with a critical observation. Identifying clinical symptoms is the first step toward determining if an MRI exam can provide clarity for better patient outcomes.
Every diagnosis starts with a critical observation. Identifying clinical symptoms is the first step toward determining if an MRI exam can provide clarity for better patient outcomes.
The Magnetic Resonance Imaging Journey
Our dedicated team ensures safety at every step
The journey from a magnetic resonance imaging (MRI) order to the final report is multifaceted and involves a high degree of collaboration and attention to detail. The goal is to safely perform a high-quality exam tailored to the patient that aids diagnosis and treatment decisions. Keeping the patient safe is paramount with steadily increasing patient volume and high demand for MRI services.
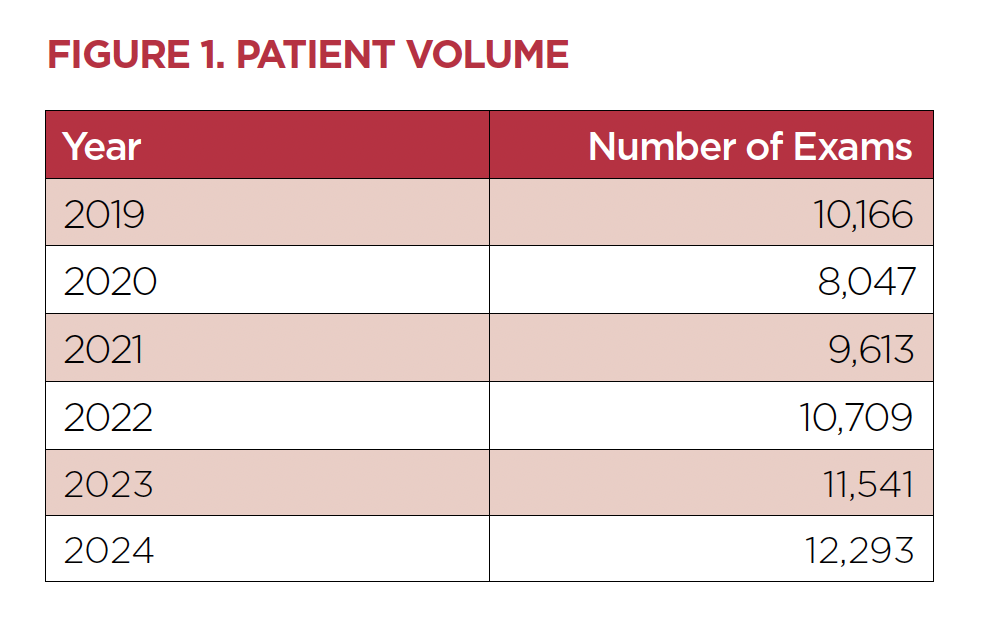
The imaging team at Children’s Wisconsin currently supports multiple MRI scanners at the Milwaukee Hospital and the New Berlin and Mequon clinics. Patient and exam volumes have shown continued growth over the past five years (Figure 1).
Ordering MRI
The journey begins when a provider identifies a patient with specific clinical symptomology who could benefit from an MRI exam. Clinical decision support tools are built into Epic to help guide referring providers. Our radiologists, MRI technologists and nurses are also available to answer questions about which exam to choose. After the order is entered, an imaging nurse completes an initial review of the patient’s needs related to sedation and which location(s) would best serve the patient. Order notes are ultimately shared with the Central Scheduling team before they contact the family to schedule the appointment.
When the appointment is scheduled, it immediately populates an Epic work queue monitored by our nurses. The nurses dive deeper into the patient’s history to determine if sedation is needed. They also research MRI safety risks by reviewing the answers to the safety-focused order questions, implant records and surgical history. Based on the patient’s age and scope of imaging needed, we may partner with child life specialists to help prepare the patient for the MRI scan and reduce the need for sedation. Otherwise, when applicable, we coordinate sedation with our anesthesiology department.
Ensuring a safe appointment
MRI safety events have demonstrated a direct relationship to our increase in patient volume. These events range from unexpected items in the MRI scanner room to unsafe items left on the patient. Each safety event is reviewed at the MRI Safety Committee, where our team analyzes and implements strategies to prevent future occurrences.
A newly scheduled order also populates a work list managed by the radiologists. The radiology team reviews every MRI order for appropriateness based on the provided clinical history. Dedicated imaging protocols are assigned for each specific exam type, which includes imaging parameters, contrast administration and add other study related medications.
Before the imaging appointment, the patient journey is supported by ensuring the family has pre-procedure instructions for arrival time and NPO. A combination of tools, including direct phone calls and text messages, is used to relay this information.
On the day of the exam, the patient is thoroughly assessed by the imaging nurse and MRI technologist. Each patient’s vital signs, weight, allergies, home medications and NPO status are reviewed. The exam order and protocol are also checked for accuracy. Additionally, the imaging nurse and MRI technologist partner with the radiologist to provide a risk-benefit assessment for each patient receiving contrast. Ensuring that gadolinium-based contrast is given safely and only when appropriate is a key focus of MRI safety. Patients are screened for risk factors that may contribute to renal insufficiency. When applicable, an estimated glomerular filtration rate is calculated. Although rare, patients with limited renal function may experience adverse effects from gadolinium-based contrast agents.
The next part of the journey is keeping the patient safe in the MRI scanner room. An MRI Safety Time Out is performed before each patient is transitioned through the scan room doors to ensure it is safe to enter. The patient is protected during the scan to prevent thermal and hearing-related injuries.
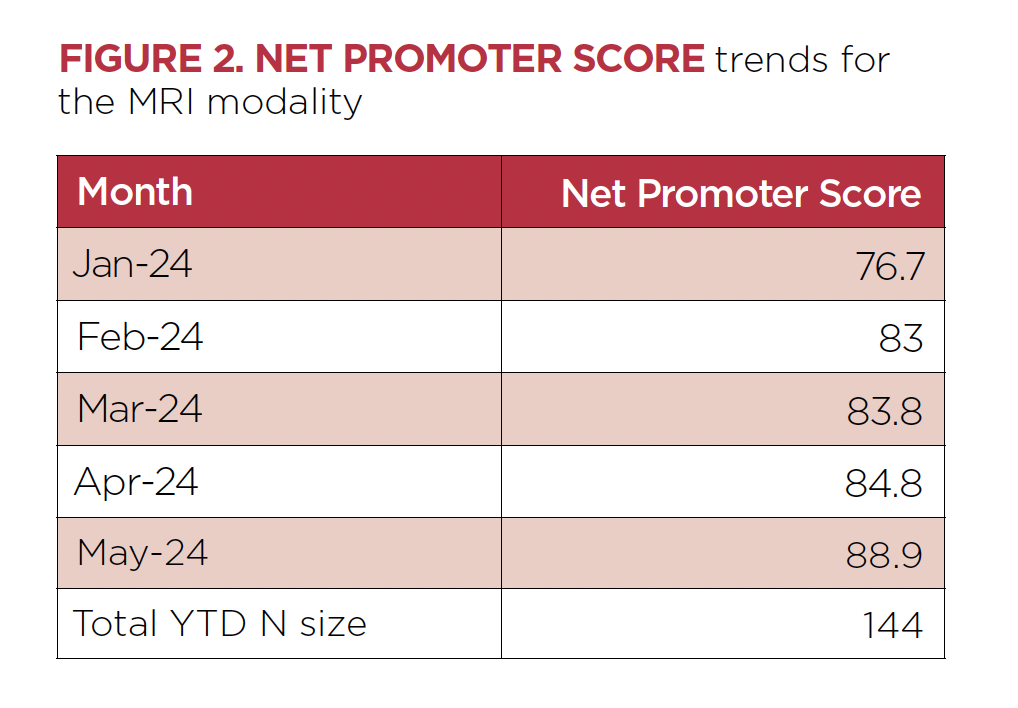
The prospect of having an MRI may induce fear and anxiety for patients and families. As demonstrated, the MRI team goes above and beyond each step to alleviate these concerns. These efforts have been recognized by a significant increase in the Net Promoter Score for the MRI modality (Figure 2).
Imaging expertise
The final step in the MRI journey is generating the imaging report. Our team of dedicated pediatric radiologists share subspecialized training in body imaging, musculoskeletal imaging, neuroradiology and interventional radiology, and work together to review each exam.
The imaging report is the final destination in a complex journey that includes the collaboration of many team members to ensure the best and safest care for every child receiving an MRI.
Kevin P. Boyd, DO
Program Director of Pediatric Body Imaging, Children’s Wisconsin, Division Chief of Pediatric Radiology and Associate Professor of Radiology, Medical College of Wisconsin
Linda Strain, BSN, RN
Imaging Manager, Children’s Wisconsin
Focusing on innovative strategies to enhance patient outcomes while reducing the need for multiple surgical interventions is always the goal in order to shape a healthier, less invasive future.
Focusing on innovative strategies to enhance patient outcomes while reducing the need for multiple surgical interventions is always the goal in order to shape a healthier, less invasive future.
Innovations in Interventional Congenital Cardiology
Expanding the range of cardiac interventions to improve patient outcomes
Most recent innovations in interventional congenital cardiology have occurred by expanding the upper and lower size limits of interventional targets. The goal has been to improve patient outcomes and to try to decrease the lifetime number of surgical interventions and their associated morbidity.
Advances in valve treatments
Interventional cardiology can now treat all four cardiac valves. Most commonly, this involves the pulmonary valve in the congenital patient population, historically with balloon dilation or stenting. Previously, surgery was the only means of valve replacement, with some patients having 10 or more sternotomies over a lifetime. The Melody® valve1 was approved in 2010 for transcatheter pulmonary valve replacement for 16-24 mm outflow diameters. Then, in 2016, the Sapien™ valve was approved for outflow tracts up to 29 mm in diameter.2 This still left many patients with no interventional options.
Enter a new generation of large outflow tract transcatheter valve systems: The Harmony® valve and Alterra Prestent® (used with the Sapien™ valve) can treat outflow tracts up to 38-40 mm in minimal diameter, with candidacy screened by preprocedural CT imaging. Adding these to our toolbelt has allowed most patients with dysfunctional right ventricle outflow tracts3 to be treated in the cardiac catheterization suite, deferring open-heart surgery.
In non-congenital structural intervention, transcatheter aortic valve replacement is mainstream, as are mitral valve clips to reduce regurgitation. There is a great deal of effort going into developing tricuspid valve regurgitation repair options with clips or implanted devices, with a huge elderly patient population. Programs are starting to explore the use of this technology in the congenital population,4 but the primary limitation is their extremely large delivery systems, often prohibitive for smaller patients. Postoperative patient anatomy can also make access to the tricuspid valve challenging. Thus far, we have succeeded in treating tricuspid bioprosthesis issues with our pulmonary valve systems, but this is a rare subset. With experience, delivery system miniaturization will likely make tricuspid and mitral valve interventions much more tenable for children.
Innovative options for neonatal care
The greatest innovations of late have been in our smallest patients: the neonates. At Children’s Wisconsin, we are currently involved in two “first in our field” randomized controlled trials involving the largest centers nationwide. The COMPASS trial compares outcomes in patent ductus arteriosus (PDA) stenting versus surgical shunt patients,5 whereas the PIVOTAL trial compares PDA device closure to standard medical therapy.6
We have developed a PDA stenting program that has increased in volume over the last five years, with the growing use of this technology for newborns who require a secure source of pulmonary blood flow as a bridge toward later repair or single ventricle palliation. While the initial results are promising,7,8 the COMPASS trial was developed to address the concern that the previous trials favorably comparing stenting to surgical outcomes may have been hampered by selection bias.
Refinements in stenting techniques
PDA stenting takes place after an echocardiogram and CT imaging shows the anatomy of the duct, which can be highly variable in length, origin, insertion and tortuosity. The approach can be from either the femoral or carotid arteries accessed by needle puncture, allowing us to place coronary stents covering the entire PDA. Post-intervention, many patients are extubated within 24 hours and have been shown to have a less challenging perioperative course compared to surgical shunt implantation. Although not without potential complications, we find most neonatal patients being referred for stenting over surgical shunting if their anatomy allows. These stents or the branch PAs being fed by them can also be further dilated, allowing for more time before the next surgical stage to get neonates home sooner, optimize the arterial growth and reduce overall morbidity.
Pioneering PDA device placement
Finally, PDA device placement is taking place in tiny patients. Previous PDA devices were approved for patients 6 kg and over. At present, the purpose-built Piccolo9 device (Figure 1) is FDA-approved for infants down to 700 g, with some interventionalists performing off-label implants in patients below that size, and there are some other devices for arterial occlusion used to further increase our ability to close PDAs in these most fragile patients. The NICU team has the choice of surgical versus device closure if medical management fails. We have worked hard to develop a program focused on the safe transfer and care of these patients in the cardiac catheterization lab and have done so in more than 100 patients weighing less than 6 kg, with our smallest patient being 700 g. We have experienced a steep learning curve. Ultimately, we may be moving this to the bedside, which has already been pioneered in some programs.
Susan R. Foerster, MD
Medical Director of Interventional Cardiology, Herma Heart Institute, Children’s Wisconsin Professor, Pediatrics, Medical College of Wisconsin
References
1. Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the US Clinical trial. J Am Coll Cardiol. Oct 27 2009;54(18):1722-9. doi: 10.1016/j.jacc.2009.06.034. PMID: 19850214.
2. Kenny D, Rhodes JF, Fleming GA, Kar S, Zahn EM, et al. 3-Year Outcomes of the Edwards SAPIEN™ Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position from the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc Interv. Oct 8 2018;11(19):1920-1929. doi: 10.1016/j.jcin.2018.06.001. PMID: 30286853.
3. McElhinney DB, Gillespie MJ, Aboulhosn JA, Cabalka AK, Morray BH, et al. Transcatheter Pulmonary Valve Replacement with the Harmony Valve in Patients Who Do Not Meet Recommended Oversizing Criteria on the Screening Perimeter Plot. Circ Cardiovasc Interv. May 2024; 17(5):e013889. doi: 10.1161/CIRCINTERVENTIONS.123.013889. Epub Apr 12 2024. PMID: 38606564.
4. Jolley MA, Sulentic A, Amin S, Gupta M, Ching S, et al. Introduction of transcatheter edge-to-edge repair in patients with congenital heart disease at a children’s hospital. Catheter Cardiovasc Interv. Feb 2024;103(2):326-334. doi: 10.1002/ccd.30935. Epub Dec 27 2023. PMID: 38149722; PMCID: PMC10911413.
5. (2023, April 14). Comparison of Methods of Pulmonary Blood Flow Augmentation in Neonates: Shunt Versus Stent (The COMPASS Trial) (COMPASS). ClinicalTrials.gov. clinicaltrials.gov/study/NCT05268094.
6. (2024, February 7). Preliminary Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Low-weight Infants (PIVOTAL). ClinicalTrials.gov. clinicaltrials.gov/study/NCT03982342.
7. Bentham JR, Zava NK, Harrison WJ, Shauq A, Kalantre A, et al. Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates with Duct-Dependent Pulmonary Blood Flow: Associations with Clinical Outcomes in a Multicenter National Study. Circulation. Feb 2018 6;137(6):581-588. doi: 10.1161/CIRCULATIONAHA.117.028972. Epub Oct 30 2017. PMID: 29084734.
8. Glatz AC, Petit CJ, Goldstein BH, Kelleman MS, McCracken CE, et al. Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants with Ductal-Dependent Pulmonary Blood Flow: Insights from the Congenital Catheterization Research Collaborative. Circulation. Feb 6 2018;137(6):589-601. doi: 10.1161/CIRCULATIONAHA.117.029987. Epub Oct 17 2017. PMID: 29042354.
9. Morray BH, Sathanandam SK, Forbes T, Gillespie M, Berman D, et al. 3-year follow-up of a prospective, multicenter study of the Amplatzer Piccolo™ Occluder for transcatheter patent ductus arteriosus closure in children ≥ 700 grams. J Perinatol. Oct 2023;43(10):1238-1244. doi: 10.1038/s41372-023-01741-1. Epub Aug 16 2023. PMID: 37587183; PMCID: PMC10541325.
Eleanor’s journey with PHVD As an infant, Eleanor required neurosurgical intervention for PHVD. Now she is 7 years old. She loves to try new things and brings joy to her friends and family.
Eleanor’s journey with PHVD As an infant, Eleanor required neurosurgical intervention for PHVD. Now she is 7 years old. She loves to try new things and brings joy to her friends and family.
The Burden of Brain Bleeds for the Premature Infant
Embracing collaborative care for infants with post-hemorrhagic ventricular dilation
Despite advances in the medical care of extremely preterm infants, intraventricular hemorrhage (IVH) remains a significant problem. Post-hemorrhagic ventricular dilation (PHVD) is a common sequela of IVH and is a significant cause of morbidity, mortality and developmental delay. Nearly 10% of all infants with IVH and 20% of infants with severe IVH will develop progressive PHVD requiring neurosurgical diversion of cerebrospinal fluid. The timing of neurosurgical intervention has traditionally relied heavily upon clinical factors, including increasing head circumference, splaying of cranial sutures, full fontanelle and subjective assessments of ventricular size on neuroimaging.
The risk of a poor neurodevelopmental outcome is significantly higher when severe IVH is complicated by PHVD, particularly for those infants who require neurosurgery. Recent work from large, randomized trials has revealed that earlier interventions using neuroimaging thresholds before developing clinical symptoms of increased intracranial pressure may lead to better long-term outcomes. Our group is striving to mitigate the impact of severe IVH on the infants cared for at Children’s Wisconsin by ensuring timely intervention for infants with PHVD.
Standardizing PHVD management
In 2017, we brought together neonatology, neurology, neurosurgery and neuroradiology to form the Neonatal Neurocritical Care Program at Children’s Wisconsin. The program was tasked with evaluating emerging evidence in the care of neonates with neurologic concerns and developing guidelines reflective of best practices.
In 2020, the program developed institutional guidelines that standardized imaging, monitoring and engagement of consulting services for infants with severe IVH at risk for PHVD. These guidelines introduced a standard measure of ventricular dilation on cranial ultrasound, frontotemporal horn ratio (FTHR). FTHR can be assessed using a single image of the cranial ultrasound (Figure 1). It is excellently concorded with MRI assessment of ventricular dilation and is easy to learn. We selected an FTHR consistent with moderate PHVD as a threshold for consideration of neurosurgical intervention.
Sharing our guidelines
While the Neonatal Neurocritical Care Program worked to improve the care of patients with PHVD within our Neonatal Intensive Care Unit (NICU), we recognized that many of the infants receiving treatment for PHVD came to us as referrals from our community partners. To provide the best and safest care to the Wisconsin neonates, we needed to spread our guidelines beyond the walls of our hospital. In 2021, we engaged with three of our largest referral sites to evaluate the possibility of sharing our internal guidelines. After securing initial interest, we facilitated communication between our neuroradiologists and the staff in the community to implement FTHR measurements on neonatal cranial ultrasounds. Our efforts quickly spread from those initial sites to include most of our referral hospitals by the end of 2022.
Collaboration on the management of PHVD has proven to be a success story in engaging with our community partners. Neonatologists in the community appreciate having an objective measure to guide escalation in care and to use in discussions with families. When transfers are needed, FTHR is an easily communicated metric to express the urgency of neurosurgical evaluation.
Our efforts in caring for infants with PHVD have been broadened beyond the regional level. Within the Children’s Hospitals Neonatal Consortium (CHNC), a collaboration of level IV NICUs across the United States and Canada, our team members hold leadership positions in focus groups interested in caring for infants with PHVD and their long-term follow-up. We collaborated on developing a workshop at a recent CHNC symposium to discuss our approach to PHVD management and share what we have learned with other institutions.
A future for infants with PHVD
Care for infants with PHVD does not stop at NICU discharge. We know these patients remain at high risk for neurodevelopmental delay, and close multidisciplinary follow-up is essential. Our Developmentally Ready Engagement for Achievement of Milestones (DREAM) clinic is designed to support these infants and their families. The clinic brings together neonatologists, neurosurgeons, neurologists, neuropsychologists, physiatrists, psychologists, palliative care and social workers to create a one-stop shop where families can better understand their infant’s developmental progress and access support as necessary. The DREAM clinic focuses on developmental catch-up in these premature infants and helps families understand their child’s developmental trajectory and personalized goals.
Susan S. Cohen, MD
Neonatologist, Children’s Wisconsin Professor of Pediatrics, Medical College of Wisconsin
Alicia J. Sprecher, MD
Neonatologist, Children’s Wisconsin Assistant Professor of Pediatrics, Medical College of Wisconsin
Publications
1. Cohen S, Flibotte J. Treatment of Posthemorrhagic Hydrocephalus. Clin Perinatol. 2022 Mar;49(1):15-25. doi: 10.1016/j.clp.2021.11.002. PubMed PMID: 35209998.
2. Sprecher A, Adams S, Cabacungan E, Carlton K, Deshmukh T, Foy A, Hokenson M, Maheshwari M, Murphy D, Powell M, Seeger Langlais K, Cohen S. Use of standard assessment of post-hemorrhagic ventricular dilation to improve collaboration with referring centers. J Perinatol. 2024 Oct 15;. PubMed PMID: 39406942.
3. Serebin M, Zhang J, Yan K, Cabacungan E, Deshmukh T, Maheshwari M, Foy A, Cohen S. Prediction of short- and long-term outcomes using pre-operative ventricular size in infants with post-hemorrhagic ventricular dilation. Childs Nerv Syst. 2024 Jul;40(7):2061-2069. PubMed PMID: 38532147.
4. Carlton K, Adams S, Fischer E, Foy A, Heffelfinger A, Jozwik J, Kim I, Koop J, Miller L, Stibb S, Cohen S. HOPE and DREAM: A Two-Clinic NICU Follow-up Model. Am J Perinatol. 2024 May;41(S 01):e1570-e1574. PubMed PMID: 36918156.
5. Cabacungan E, Adams S, Best B, Foy AB, Singh A, Cohen SS. Variability in neurosurgical management and associated comorbidities and complications among preterm patients with posthemorrhagic hydrocephalus in the United States. J Neurosurg Pediatr. 2023 Mar 10;:1-8. PubMed PMID: 36905669.
Comprehensive Care for Children with Spina Bifida
How the Spina Bifida Program has supported health and independence for more than 50 years
Spina bifida is described as the most complex congenital condition compatible with life.1 Myelomeningocele is the most severe form of spina bifida. It causes anomalies in the brain and spinal cord that may result in hydrocephalus, neurogenic bowel and bladder, lower extremity paralysis, scoliosis and other orthopedic concerns.2 The Spina Bifida Program at Children’s Wisconsin was established more than 50 years ago to promote collaboration among the specialists necessary to manage spina bifida. The Spina Bifida Program is led by the Neurosciences Center at Children’s Wisconsin and includes about 25 clinicians from neurosurgery, orthopedic surgery, neuropsychology and physical medicine and rehabilitation. The Spina Bifida Program has evolved, but its mission remains constant: to promote health and enhance the quality of life for children and youth with spina bifida.
Art and science of care
We often find that caring for children with spina bifida is both an art and a science due to the unique complexities of each patient. Predicting a patient’s condition trajectory, cognitive ability and functional outcomes over the lifespan is impossible. The size and location of the spina bifida lesion provide some indication, yet there is a wide range of outcomes. Social determinants of health also significantly impact outcomes in patients with spina bifida, beginning with access to prenatal care and referral to a fetal care center for accurate counseling about spina bifida.
Although there is no cure for spina bifida, enhanced management and prevention of secondary conditions have dramatically increased the lifespan of people with spina bifida. Most people with spina bifida live well into adulthood, and some reach senior age. Routine exams, surveillance imaging (including MRI, fluoroscopy, ultrasound and X-ray) and ongoing therapies preserve functional ability and assist patients in achieving independence. The Spina Bifida Program coordinates care to ensure patients complete necessary imaging and follow-up appointments by provider recommendations. The approximately 250 patients in the Program generate 1.5% of the total ambulatory imaging volume at Children’s Wisconsin.
Addressing surgical needs
Surgical intervention is a significant aspect of spina bifida care, beginning with neurosurgical closure of the myelomeningocele. Other common surgeries are:
• Procedures to address hydrocephalus
• Bladder reconstruction
• Creation of continent channels for bowel and bladder management
• Spinal fusion
• Orthopedic procedures to correct foot deformities and promote alignment to support ambulation
Last year, our team performed 135 surgeries on patients with spina bifida. At Children’s Wisconsin, surgical recommendations for patients with spina bifida are made with input from multiple specialists within the care team. The Spina Bifida Program fosters the collaboration necessary to provide the best and safest care for individuals with spina bifida.
Improving long-term outcomes
In addition to clinical care, members of the spina bifida team are also involved in research to improve health outcomes for youth with spina bifida. Our Spina Bifida Program is one of only seven clinics in the United States funded by the Centers for Disease Control and Prevention to participate in the National Spina Bifida Patient Registry and the Urologic Management to Preserve Initial Renal Function Protocol for Young Children with Spina Bifida (UMPIRE).2
These projects collect longitudinal data on a large sample of individuals with spina bifida to study the effectiveness of various interventions and support clinical care guidelines. Other spina bifida research led by Children’s Wisconsin explores body composition and energy expenditure, sociodemographic disparities that impact access to prenatal care and interventions, exploration of quality of life for youth and families, methods to enhance cognitive growth and self-management in adolescents and young adults. Children’s Wisconsin collaborates with other spina bifida centers across the United States and actively participates in workgroups organized by the National Spina Bifida Association.
A lifelong effort
Comprehensive spina bifida care is more than a series of clinic visits. It is a coordinated effort throughout the lifespan that promotes physical and mental health within individual goals and values. Care is evidence-based and delivered within spina bifida practice guidelines. The Neurosciences Center at Children’s Wisconsin is proud to lead the world-class Spina Bifida Program that empowers children and youth with spina bifida to achieve their fullest potential.
References
1. Spina Bifida Association. What is Spina Bifida? Updated 2024. Accessed June 18, 2024. spinabifidaassociation.org.
2. U.S. Centers for Disease Control and Prevention. Spina Bifida. Updated 2024. Accessed June 18, 2024. cdc.gov/spina-bifida/index.html.

In 2023, we performed nearly 900 in-person and telemedicine exams. These screenings resulted in 15 infants treated with intravitreal injections and 43 infants treated with laser.
In 2023, we performed nearly 900 in-person and telemedicine exams. These screenings resulted in 15 infants treated with intravitreal injections and 43 infants treated with laser.
Vision for Life
A proactive approach to eye care prevents blindness for our youngest patients
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness that develops in children born at significantly young gestational ages or low birth weights. While normal blood vessel growth typically progresses in utero from the posterior pole to their final position near the ora serrata, premature infants often arrest in this development, resulting in the formation of progressive scar tissue and neovascularization. When left unchecked, the course of ROP results in retinal detachment and permanent vision loss. Appropriate timing of exams and treatment is crucial.
The Eye Program at Children’s Wisconsin takes a proactive approach to managing ROP. By following ROP with sequential eye exams, we can intervene with treatments before retinal detachment develops.
ROP exams and treatment
Children born ≤30 weeks and ≤1500 grams are screened for the presence of ROP. Some infants follow a benign growth pattern and are followed with eye exams every two to three weeks until retinal maturity. Other infants must be seen weekly or more often to ensure treatment is provided at the appropriate time.
In acute phases of the disease, the first treatment often is an anti-vascular endothelial growth factor (VEGF) medication (e.g., bevacizumab or aflibercept) injected into the eye. This helps to acutely decrease the growth of abnormal blood vessels and scarring and has been shown to allow for the growth of the eye’s natural blood vessels. The second treatment option uses a laser (pan-retinal photocoagulation) to permanently ablate the avascular retina, which drives the production of scar tissue and neovascularization. Given the possible recurrence of ROP following anti-VEGF injections, laser treatment is also conducted to finalize an infant’s treatment.
Our team of five ROP specialists provides in-person exams for infants at every Level 3 NICU in the Milwaukee metro area and telemedicine services in other areas of the state.
In 2023, we performed nearly 900 in-person and telemedicine exams. These screenings resulted in 15 infants treated with intravitreal injections and 43 infants treated with laser.
The importance of follow-up
A major challenge of compliance with ROP screening occurs when families transition from inpatient to outpatient follow-up. If the retinal vessels have not fully matured at discharge from the NICU, infants require eye exams every one to three weeks. This can be a demanding schedule for parents, and the exams can be stressful for them to watch. If a family does not keep their ROP appointments, the child may be at risk for permanent vision loss.
Some studies report missed appointment rates for ROP ranging from 14% to 25.5%.1, 2 Our rate was approximately 19% over a three-month period. To address missed ROP appointments, we implemented a tracking system in the NICUs and our clinics and collaborated with transportation and social services to support timely visits.
Further research shows that many parents do not adequately understand ROP screening and the importance of frequent follow-up.3 The few educational tools available are insufficient or at a high reading level.4
We created an educational video about ROP and the exam process to show parents in our clinics. We introduced the video in March 2023 and offered it to 83 families. Of the 67 families who opted to watch it, 19 (28%) responded to a survey about the video. Survey results showed that the video helped improve understanding of the necessity of exams, and 83% of survey respondents stated that the video significantly improved their understanding of the importance of follow-up exams. No-show rates three months after the intervention decreased to 14%. This was not a statistically significant difference and cannot be solely attributed to the video, but it does show a promising trend.
We presented this project nationally at the American Association for Pediatric Ophthalmology and Strabismus meeting in April 2024 and received excellent feedback. We hope to continue educating our community and improving the outpatient follow-up care for ROP in premature infants.
Alex Khammar, MD
Medical Director of Pediatric Ophthalmology, Children’s Wisconsin
Associate Professor of Ophthalmology, Medical College of Wisconsin
Heather Stiff, MD
Pediatric Ophthalmologist, Children’s Wisconsin
Assistant Professor of Ophthalmology, Medical College of Wisconsin
Jacob Martin, MD
Pediatric Ophthalmologist, Children’s Wisconsin
Assistant Professor of Ophthalmology, Medical College of Wisconsin
References
1. Santineau K, Abikoye TM, Guerin CM. Determinants of compliance with outpatient examinations for infants at risk for retinopathy of prematurity. J AAPOS. Jan 17 2024:103813. doi: 10.1016/j.jaapos.2023.11.015. Epub ahead of print. PMID: 38242229.
2. Law C, Yu CW, Hawley GD, Manickavachagam K, Hopman WM, Strube YNJ. Missed appointments in a tertiary academic pediatric ophthalmology and adult strabismus service: cross-sectional study and literature review. J AAPOS. Apr 2023;27(2):77.e1-77.e6. doi: 10.1016/j.jaapos.2023.01.012. Epub Feb 28 2023. PMID: 36863683.
3. Eneriz-Wiemer M, Liu SD, Chu MCY, Uribe-Leitz T, Rajani K, Sankar M, Robbins SL, Lee HC, Woodard C, Wang CJ. Parents’ Knowledge and Education of Retinopathy of Prematurity in Four California Neonatal Intensive Care Units. Am J Ophthalmol. Jul 2018;191:7-13. doi: 10.1016/j.ajo.2018.03.039. Epub Apr 3 2018. PMID: 29621506.
4. Yilmaz FH, Tutar MS, Arslan D, Çeri A. Readability, understandability, and quality of retinopathy of prematurity information on the web. Birth Defects Res. Jul 15 2021;113(12):901-910. doi: 10.1002/bdr2.1883. Epub Feb 17 2021. PMID: 33594835.
Our conservative care approach to patients with scoliosis utilizes the most advanced and innovative technologies available.
Our conservative care approach to patients with scoliosis utilizes the most advanced and innovative technologies available.
Improvement in Care for Early Onset Scoliosis
How advanced bracing technologies and holistic approaches are revolutionizing treatment for young patients
In the 1940s, a Milwaukee physician, Walter Blount, MD, developed one of the first successful methods for the conservative care of scoliosis, dubbed the “Milwaukee Brace.” This brace became the gold standard around the world in its time for preventing curve progression.
Strong interest in preventing curve progression to avoid surgical intervention continues at the AIM Spine Center at Children’s Wisconsin. Concerted efforts in treating our youngest patients with scoliosis, who are at the highest risk for life-threatening severe scoliosis, have led our center to develop bracing using surface topography, 3-D printing and sensor technology.
In conducting research to improve the treatment of early-onset patients, we considered multiple important factors:
• Typical treatment for these young patients often involved years in full-time body casts. New technology allows for the selection of curves to achieve the same correction with new bracing techniques, a much-improved quality of life, less general anesthesia and greater comfort.
• Surface topography with the patient in the corrected position gives an exact 3-D replica of the child’s torso, automatically making the brace easy to adapt.
• The AIM Spine Center was the first to use 3-D printing technology for bracing these young patients. This allows us to fabricate braces using lighter and more comfortable materials. This was done as a collaborative project with the engineers at Milwaukee School of Engineering (MSOE).
Collaborative efforts make a difference
J. Channing Tassone, MD, Benjamin Escott, MD, Xue‑Cheng Liu, MD (our director of pediatric research) and I have published and presented the results of our technique at international meetings. Our center has been invited to participate in national grants studying this issue.
We have found that the brace will only work if it is worn. All our juvenile scoliosis patients have a sensor embedded that monitors the number of hours per day. If compliance is suboptimal, the results are reviewed to see why the child is having problems with the brace.
Here are some comments from a mother regarding the brace:
“My daughter is very active, and she struggled mightily wearing the [original] brace in the beginning. It was difficult for her to maintain her same level of physical activity, and she couldn’t keep up with her peers or play with her brother the same way. It was also very uncomfortable and hot to wear during the summer. The brace would cause skin issues.
“Finding clothes that fit is always difficult. My daughter just always felt different from her peers.
“The new brace is incredible. It’s much lighter, far more comfortable and it allows her to be active, so she is much more compliant about wearing it. She also wore it through last summer, and her skin didn’t break out.”
Providing holistic support
The AIM Spine Center is one of the few scoliosis centers with a dedicated spine psychologist, Nicolas Young, PhD. This is in addition to our scoliosis support group pioneered by our nurses, Beth Wahlquist, BSN, RN, and Tracie Brasch, RN. Adjusting to the brace can be emotionally difficult, and our clinic has specific expertise to aid the child in developing a positive attitude toward the brace with no sense of awkwardness or shame. Christa Thalacker, NP, one of our nurse practitioners, has worked to implement the early-onset questionnaire as part of a database registry.
The scoliosis clinic seeks to offer the best conservative care possible. We believe it is important to combine the best expertise available in our institution with the experts in outside institutions, such as MSOE and a local 3-D printing company, to develop new scoliosis bracing materials that are more durable and comfortable for a brace designed to fit the individual child. Dr. Thometz recently visited a 3-D printing conference in Leipzig, Germany to explore the latest technologies in this rapidly developing field.
Our conservative care approach to these patients utilizes the most advanced and innovative technologies available. In addition to these technologies, Dr. Liu and Dr. Thometz are working toward obtaining a large national grant that will help us provide the best emotional and psychological support during treatment and explore new materials for improved patient comfort and quality of life. At the AIM Spine Center, we are committed to taking this collaborative approach to improve outcomes for these challenging patients.
John G. Thometz, MD
Vice Chair for Research, Orthopedic Surgery, Children’s Wisconsin, Professor of Orthopedic Surgery, Medical College of Wisconsin
The Tracheostomy Tube Journey
Supporting families and ensuring safety at every step, from placement to removal
From the day a child receives a tracheostomy tube, our team begins working on the process of getting that tube removed. At Children’s Wisconsin, the tracheostomy and home ventilator team includes a variety of specialists from around the hospital: pediatric otolaryngologists, pulmonologists, neonatologists, critical care physicians, nurse specialists, respiratory therapists, speech and language pathologists, medical social workers and others.
When a child first becomes a candidate for a tracheostomy tube at our hospital, it can be a scary and unknown world for the family. Our team’s initial focus is supporting and educating the family as they adjust to a “new normal” (Figure 1). This process includes face-to-face and hands-on education methods, all aimed at instilling confidence in the family’s abilities to care for their child after discharge or what we call the “Stepping Stones to Home.”
The critical first week
While daunting for the family, the first week after placing a fresh tracheostomy can also be one of the more challenging times for the care team. We place a bedside sign next to every patient with specific information about the trach tube that can be quickly accessed in an emergency. While the fresh stoma is healing, accidental decannulation (removal of the trach) can occur, resulting in difficulty ventilating the child. We also watch for wound problems, like breakdown or granulation. To minimize these occurrences, it is often imperative to maintain a high level of sedation and even paralysis through pharmacologic means for up to seven days until the first tracheostomy tube change.
Our team recently studied the effect of an earlier first tube change (day five or earlier) on safety and complications. We found that earlier tube changes are not associated with any increase in complications or wound problems, but there is a decrease in the requirement for some of the sedative medications. These findings shifted our practice on the timing of first trach changes so that we can minimize the difficult time that a family has to wait until they can interact with their child again.
Addressing issues
Once home, patients receive routine follow-up care in our multidisciplinary tracheostomy/home ventilator clinic, where the team not only works on weaning ventilator requirements but also continues the work of facilitating speech using a one-way speaking valve and, ultimately, decannulation. During this part of the journey, the pediatric otolaryngologist may perform a surgical endoscopy to evaluate for narrowing or collapse of the airway or any other obstructing pathology (Figure 2) that may hinder the decannulation process. The surgeon may also recommend other surgeries to correct these pathologies, including adenotonsillectomy, supraglottoplasty, balloon dilation or laryngotracheal reconstruction — a surgery that augments a narrowed airway, usually with rib cartilage grafts.
Once the patient has met the criteria — off the ventilator, free of respiratory illnesses and with a non-obstructed airway — the medical team will begin the process of capping the tracheostomy tube. This occludes the surgical airway so the patient breathes in a more native state through their nose and mouth. Once the patient tolerates this during the day for extended periods, they will have an overnight sleep study with a capped tracheostomy tube. Because the tube itself occludes up to 50% of the child’s airway and can cause obstructive sleep apnea by itself, our team instituted the process of removing the child’s tube and placing a small, capped tube in the airway for the sleep study. This minimizes obstruction and allows for an optimal result that predicts successful decannulation.
Navigating final steps
When patients pass their capped sleep study and meet all other criteria, they will be admitted for decannulation. This involves admission to the pediatric intensive care unit where the pediatric otolaryngology team will help the family remove the tracheostomy tube one last time — only this time, they will not replace it with a new one. A semi-occlusive dressing is placed over the stoma, and the patient is monitored overnight for any respiratory distress or desaturation. If successful, the patient is discharged home, and the team will follow them as an outpatient until the stoma has closed and the patient has reached their long-awaited goal of a trach-free life.
Tracheostomy tubes can be scary for families and providers, but they shouldn’t be. It should always be remembered that from the moment the tube is placed, the team is working hard to get it out. Our team at Children’s Wisconsin continues to streamline this process every step of the way.
Michael E. McCormick, MD
Complex Pediatric Otolaryngologist, Children’s Wisconsin, Professor of Pediatric Otolaryngology, Medical College of Wisconsin
Cecille G. Sulman, MD
Medical Director of Pediatric Otolaryngology, Children’s Wisconsin
Chief and Professor of Otolaryngology, Medical College of Wisconsin

From procedural and diagnostic excellence to continual evaluation and improvement of our care, the Multidisciplinary Thyroid Group is proud to care for children with thyroid cancer and their families.
From procedural and diagnostic excellence to continual evaluation and improvement of our care, the Multidisciplinary Thyroid Group is proud to care for children with thyroid cancer and their families.
Comprehensive Care for Pediatric Thyroid Nodules
Ensuring diagnostic accuracy and optimal treatment for thyroid nodules in children
What should you do when a child is diagnosed with a thyroid nodule? While common in adults, thyroid nodules are encountered less frequently in the pediatric population. Moreover, pediatric thyroid nodules are more often cancerous than thyroid nodules in adult patients. As such, many providers need more clarification about the path forward for these children.
At Children’s Wisconsin, the Multidisciplinary Thyroid Group seeks to provide the best and safest care for children with thyroid nodules and cancer. Utilizing the American Thyroid Association’s (ATA) guidelines for the management of pediatric patients with thyroid nodules and cancer, we have brought together specialists from different areas within Children’s Wisconsin — endocrinology, surgery, otolaryngology, pathology, radiology and oncology — who have expertise in diagnosing and treating children with thyroid cancer.
Expertise and precision
Our Multidisciplinary Thyroid Group offers unique services to children and their families, one of which is a fine needle aspiration (FNA) biopsy for the diagnosis of thyroid cancer. Our FNA diagnostic services are provided exclusively by interventional radiologists and cytopathologists with expertise in caring for children. Our pediatric anesthesiology team is available to support our patients who require sedation, though many children tolerate this minimally invasive procedure well with little pain while awake.
Ensuring diagnostic adequacy and accuracy is at the heart of the thyroid FNA program at Children’s Wisconsin. All FNAs are performed under ultrasound guidance (as recommended by the ATA) by pediatric-trained interventional radiologists.
In addition, one of two board-certified pediatric cytopathologists attends every FNA procedure, with preliminary specimen processing and review occurring in the interventional radiology suite. This on-site adequacy evaluation by pediatric cytopathologists is a highly specialized service performed at only a handful of pediatric hospitals nationwide. It allows us to ensure that a diagnosis can be made based on the cells obtained during the biopsy, which in turn obviates the need for additional diagnostic FNA procedures.
Diagnostic accuracy
Pediatric thyroid FNA adequacy ranges from approximately 74% to 85%, depending upon the institution1,2. As a result of our team’s expertise and rapid on-site specimen adequacy assessment practice, Children’s Wisconsin has a diagnostic adequacy rate for thyroid FNAs of nearly 97%.
Thyroid FNA diagnostic classification includes two “indeterminate” diagnostic categories with some features of malignancy but insufficient evidence to support a cancer diagnosis. The risk of cancer among pediatric patients who receive an “indeterminate” cytologic diagnosis on FNA ranges widely from 30% to 70%. Because of this variable but significant risk of malignancy, the ATA recommends that this subset of patients undergoes surgery without a definitive diagnosis of cancer.
Indeterminate diagnoses constitute approximately 20% of pediatric FNA diagnoses,3 prompting surgical intervention that may not be necessary. In contrast, at Children’s Wisconsin, the thyroid FNA indeterminate rate is only 8.6%. Of those patients with an indeterminate diagnosis at Children’s Wisconsin, nearly 43% were diagnosed definitively with thyroid cancer at the time of surgery.
Innovative thyroid cancer care
In addition to developing a strong thyroid FNA program since establishing the Multidisciplinary Thyroid Group in 2016, we’ve developed and implemented multiple initiatives to continue improving the care of our thyroid cancer patients. We review patient medical reports at our biweekly tumor board to identify which patients have nodules that should undergo FNA, preventing unnecessary procedures and accelerating care for patients in which there is a high suspicion of cancer. To help alleviate the hypocalcemia that can occur after surgery, we developed updated calcium monitoring protocols for patients who are post‑thyroidectomy.
Because some thyroid cancers may be associated with heritable cancer disorders, we have incorporated oncologists with expertise in cancer predisposition syndromes into our multidisciplinary group. We are reviewing, revising and updating our radioactive iodine (RAI) guidelines and educational literature for families, as RAI is an important form of treatment for patients with more aggressive thyroid cancer. In the last year, we became a participant in a multi-institutional cooperative study examining the molecular profile of pediatric thyroid cancer. This will, in turn, help identify potential therapeutic targets and prognostic markers for children with thyroid cancer.
From procedural and diagnostic excellence to continual evaluation and improvement of our care, the Multidisciplinary Thyroid Group is proud to care for children with thyroid cancer and their families.
Lauren N. Parsons, MD
Pediatric Pathologist, Children’s Wisconsin, Associate Professor of Pediatric Pathology, Medical College of Wisconsin
References
1. Cherella CE, et al. Differences in Thyroid Nodule Cytology and Malignancy Risk Between Children and Adults. Thyroid. Aug 2019;29(8):1097-1104.
2. Amirazodi E, et al. Pediatric thyroid FNA biopsy: Outcomes and impact on management over 24 years at a tertiary care center. Cancer Cytopathol. Nov 2016;124(11):801-810.
3. Wang H, et al. Incidence and malignancy rates of indeterminate pediatric thyroid nodules. Cancer Cytopathol. Apr 2019;127(4):231-239. doi: 10.1002/cncy.22104. Epub Feb 15 2019.
Our pediatric transplant team aims to enhance vaccination rates and ensure durable and long-term immunity among our liver transplant population.
Our pediatric transplant team aims to enhance vaccination rates and ensure durable and long-term immunity among our liver transplant population.
The Critical Importance of Immunization
Children’s Wisconsin is helping to increase vaccination rates following liver transplantation
Liver transplantation is a life-saving intervention for children with severe liver disease. However, many of these transplants occur at a young age. Therefore, most patients haven’t completed their primary vaccination series. Historically, the American Society of Transplantation (AST) and the American Academy of Pediatrics (AAP) advised against live vaccines for four weeks pre-transplant and indefinitely post-transplant due to the risk of severe infections and/or rejection in immunocompromised patients.1
This was a cautious approach given the complexities of caring for transplant recipients. However, declining immunization rates in the general population compromise herd immunity, leaving transplant recipients increasingly vulnerable to vaccine-preventable illnesses. It’s estimated that 16% of pediatric transplant patients are hospitalized due to vaccine-preventable illnesses, and even more are treated for such infections in an outpatient setting.2
Given this shifting landscape, the AST has updated its guidelines to permit live vaccination in carefully selected post-transplant patients,1 acknowledging that the benefits of vaccination may now outweigh the risks for some individuals. While single-center studies support the safety and efficacy of post-transplant live vaccination, further research is needed on long-term immunity to live and inactivated vaccines in this population, including the optimal timing and scheduling of vaccinations.3-8
Increasing vaccination rates
Our pediatric transplant team aims to enhance vaccination rates and ensure durable and long-term immunity among our liver transplant population. Therefore, our multidisciplinary team of hepatologists, infectious disease providers, nurse practitioners, pharmacists, transplant coordinators and fellows established immunization eligibility criteria for live and inactivated vaccines (Table 1). During annual post-transplant visits, our team worked to ensure families were educated about new recommendations, and that titers were obtained for the following: varicella, measles, mumps, rubella, Haemophilus influenzae B (HiB), hepatitis A (HAV) and hepatitis B (HBV).
Based on titer levels and eligibility criteria, personalized recommendations were communicated to families and primary care providers. Families decided to vaccinate in consultation with their health care teams, administering these vaccines at their primary care offices. Potential side effects were discussed, and follow-up occurred four weeks post-vaccination to monitor for any adverse events. Barriers to vaccination were explored for patients who did not receive the recommended vaccinations to identify areas for improvement.
Improving immunity
Fifty-four pediatric liver transplant patients participated in at least three out of four Plan Do Study Act (PDSA) cycles. By the end of cycle four, both vaccination rates and immunity levels improved. The number of patients receiving recommendations for live and inactive vaccines varied across PDSA cycles.
A small portion (11%) of the participants were immune to live and inactive vaccines throughout the four cycles. Another 18.5% were not eligible for live vaccinations. Most (76%) of those eligible received at least one live vaccine, and nearly all (96.5%) obtained subsequent titers. All patients achieved immunity to measles (100%), and nearly all achieved immunity to mumps, rubella and varicella at 95%, 95% and 94%, respectively.
Longitudinal titer assessment revealed a 42% loss of immunity to live vaccines over time, primarily varicella (87%) and, to a lesser extent, measles (26%). This information is crucial for establishing an evidence-based immunization schedule for post-transplant patients and highlights the need for ongoing monitoring and potential boosters.
Continual analysis and counseling
We aim to simplify the vaccination process by administering recommended vaccines in our liver transplant clinic, reducing the burden of multiple visits for families. Ongoing analysis of our results will refine counseling for patients and families, providing them with the most up-to-date information. As new guidelines evolve, we are committed to adapting our approach to provide optimal protection for our transplant population.
Lama Alibrahim, MD
Resident, Marshfield Clinic
Bernadette E. Vitola, MD, MPH
Pediatric Transplant Hepatologist, Children’s Wisconsin, Associate Professor, Pediatric Hepatology, Medical College of Wisconsin
Alexis J. Gumm, MD
Pediatric Gastroenterologist and Pediatric Transplant Hepatologist, Children’s Wisconsin
Assistant Professor, Pediatric Gastroenterology, Medical College of Wisconsin
Stacee M. Lerret, PhD, APNP
Pediatric Gastroenterology Nurse Practitioner, Children’s Wisconsin Professor, Pediatric Gastroenterology, Medical College of Wisconsin
References
1. Danzinger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009 Dec;9 Suppl 4:S258-62. doi: 10.1111/j.1600-6143.2009.02917.x. PMID: 20070687.
2. Brady MT, Jackson MA, Long SS, Kimberlin DW. Red Book: 2018–2021 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; 2018.
3. Feldman AG, Sundaram SS, Beaty BL, Kempe A. Hospitalizations for respiratory syncytial virus and vaccine-preventable Infections in the first 2 years after pediatric liver transplant. J Pediatr. 2017;182:232-238. doi.org/10.1016/j.jpeds.2016.12.021.
4. Feldman AG, Beaty BL, Curtis D, Juarez-Colunga E, Kempe A. Incidence of hospitalization for vaccine-preventable infections in children following solid organ transplant and associated morbidity, mortality costs. JAMA Pediatrics. 2019;173:260-268. doi.org/10.1001/jamapediatrics.2018.4954.
5. Feldman AG, Hsu EK, Mack CL. The Importance of Prioritizing Pre and Posttransplant Immunizations in an Era of Vaccine Refusal and Epidemic Outbreaks. Transplantation. 2020 Jan;104(1):33-38. doi: 10.1097/TP.0000000000002936. PMID: 31876696; PMCID: PMC6936334.
6. Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13563. doi.org/10.1111/ctr.13563.
7. Kawano Y, Suzuki M, Kawada J, et al. Effectiveness and safety of immunization with live-attenuated and inactivated vaccines for pediatric liver transplantation recipients. Vaccine. 2015;33(12):1440-1445. doi.org/10.1016/j.vaccine.2015.01.075.
8. Khan S, Erlichman J, Rand EB. Live virus immunization after orthotopic liver transplantation. Pediatr Transplant. 2006 Feb;10(1):78-82. doi: 10.1111/j.1399-3046.2005.00403.x. PMID: 16499592.
Robotic Laparoscopic Surgery in Pediatric Urology
Revolutionizing pediatric care with minimally invasive techniques and expert surgical teams at Children’s Wisconsin
Laparoscopic surgery has been used in pediatric surgery since the 1970s.1 Initially, it was employed for diagnostic purposes. The first laparoscopic surgical intervention was a laparoscopic cholecystectomy performed in 1985, soon followed by a nephrectomy in 1990.2,3 In a short period, laparoscopic surgery, also referred to as minimally invasive surgery (MIS), has transformed the treatment of surgical disease. MIS has become the standard of care for numerous surgical conditions in pediatric and adult patients.
Laparoscopic surgery involves making only a single or a few small incisions under 1 centimeter in length to allow the endoscope (camera) and instruments to be placed within the body to perform a procedure. Since MIS can be performed through small incisions, it offers several advantages to patients compared to open surgery. These benefits include a decrease in postoperative pain, less narcotic use, improved cosmesis, shorter hospital stay and quicker return to full activity versus most open surgery. Numerous studies have demonstrated that MIS is as effective and safe as open surgery in children.
Rise of robotic-assisted surgery
In 1998, Intuitive Surgical Inc. developed and released the first da Vinci Surgical System. Although the da Vinci Surgical System is referred to as a “robotic system,” it is, in fact, a robotic-assisted laparoscopic surgery system because it cannot function independently and is completely controlled by the surgeon. The robotic system was developed to overcome some limitations of conventional laparoscopy, including two-dimensional visualization and instrument articulation limited by the surgeon’s wrist. These limitations increase the technical difficulty of laparoscopy in some situations when sewing and tying sutures.
Robotic-assisted laparoscopic surgery quickly gained prominence because of the system’s benefits for adult and pediatric laparoscopic procedures. The robotic system allows for three-dimensional visualization, visual magnification, removal of any physiologic tremor from the surgeon and improved instrument dexterity. These instruments allow for 90-degree articulation, much like the human wrist. These benefits allow for easier suturing, knot tying, tissue dissection and manipulation, making this technology ideal for pediatric urologic reconstructive surgery. The next generation of robotic systems is expected to miniaturize the size of incisions to make it more broadly competitive with the laparoscopic approach.
Advantages in pediatric urology
Robotic-assisted laparoscopic surgery has now become the new gold standard for adult urology for prostatectomies and kidney surgeries. Pediatric urologic surgery that used to be only performed as open surgical repairs can now be safely performed with similar and possibly even better results, along with providing the benefits of MIS with this robotic technology. Patients now routinely return home the day following surgery with limited discomfort, compared to several days and more discomfort when the same procedure is performed in an open fashion.
Our department currently offers robotic-assisted laparoscopic surgery for children with various conditions. The most common robotic-assisted laparoscopic surgeries in pediatric urology include pyeloplasty for ureteropelvic junction obstruction, which affects kidney drainage, ureteral reimplantation for vesicoureteral reflux, nephrectomy, nephroureterectomy, bladder surgery and genitourinary reconstructive surgery.
Our expertise
Robotic-assisted laparoscopic surgery and MIS illustrate how technology has transformed and improved the surgical treatment and approach to certain surgical conditions. It is imperative to have a surgical team with experience caring for and treating both pediatric conditions and patients to have safe and successful surgical outcomes in pediatric patients. At Children’s Wisconsin, we have a dedicated robotic surgery program and team with our own da Vinci Surgical System. Our robotic and MIS team consists of dedicated professionals specially trained in caring for pediatric surgical patients and diseases.
Our operating room team consists of specialized nurses, anesthesiologists and four fellowship-trained Pediatric Urologists (Elizabeth Roth, MD, Jonathan Ellison, MD, Doug Storm, MD, and myself) who have all been trained in robotic-assisted laparoscopic surgery and the care of pediatric urologic conditions. The first robotic-assisted laparoscopic procedure was performed at Children’s Wisconsin in March 2012, and since that time, we have successfully performed more than 300 more.
At Children’s Wisconsin, we continue to strive to provide the best care possible for all our patients. We do this by providing access to the benefits of new technology in MIS and robotic laparoscopic surgery at a pediatric hospital dedicated to treating children’s unique needs. We are very excited about the future and the opportunity to continue to provide access.
Travis W. Groth, MD
Pediatric Urologist, Children’s Wisconsin, Associate Professor of Pediatric Urology, Medical College of Wisconsin
References
1. Gans SL, Berci G. Advances in endoscopy of infants and children. J Pediatr Surg, 1971. 6(2):199-233.
2. Reynolds Jr W. The first laparoscopic cholecystectomy. JSLS, 2001. 5(1): 89-94.
3. Clayman RV, et al. Laparoscopic nephrectomy: initial case report. J Urol, 1991. 146(2):278-82.

Using Quality Improvement Data to Enhance Surgical Patient Care
Data helps identify opportunities and measure success
Children’s Wisconsin is an American College of Surgeons (ACS) Verified Level 1 Pediatric Surgery Center and participates in the National Surgical Quality Improvement Program-Pediatric (NSQIP-P). NSQIP-P is a data registry that produces risk-adjusted and benchmarked reports based on individual hospitals’ surgical outcomes. Children’s Wisconsin uses these reports to identify opportunities for improvement in surgical quality and measure our progress against our peers. We are also a member hospital of several national collaboratives, including the Pediatric Surgery Quality Collaborative (PSQC).
Surgical site infections
In early 2023, the Pediatric General Surgery team at Children’s Wisconsin joined a national collaborative on surgical site infections (SSIs) in partnership with PSQC, NSQIP-P and their respective member hospitals. The objective of this effort was to substantially reduce the incidence of postoperative SSIs for pediatric patients undergoing colorectal procedures with anastomosis and abdominal closure through the use of a prescribed treatment bundle. The treatment bundle includes:
• Preoperative skin prep
• Surgical site preparation
• Intraoperative pathways
• Intraoperative patient temperature maintenance
• Instrument tray guidance around surgical wound closure
• Postoperative antibiotics and surgical site monitoring
Successful implementation of the project required collaboration and input from many departments across the hospital, including surgery, anesthesiology, nursing, surgical services staff and surgical clinical reviewers.
To operationalize the project, we took the following actions:
• Built a standard operative note template
• Educated surgeons, operating room staff and postsurgical care areas on
the enhanced operating room procedures
• Developed a process for loop closure and feedback on compliance
After approximately nine months of data collection, our report showed that patient temperature often fell below 36 degrees Celsius for more than 30 minutes and sometimes rose above 38 degrees Celsius for more than 30 minutes. These findings are meaningful, as studies have shown that intraoperative hypothermia and hyperthermia are associated with increased SSIs.
In response, we partnered with our colleagues in Anesthesia to raise awareness of the bundle elements and discuss opportunities to standardize room temperature during surgical cases and room turnover. Our data also reflected inconsistency in charting wound assessments and changing dressings at 48 hours. So, we developed a new process for wound care, bathing and dressing changes.
Expected impact
Data was collected by NSQIP-P throughout 2024 and analyzed by PSQC quarterly. Based on this data, our goals are to increase compliance with the bundle elements, increase documentation of adherence to the bundle elements within operative notes, promote intraoperative normothermia among surgical teams and monitor how these initiatives impact SSI rates.
Dave R. Lal, MD, MPH
Chief, Division of Pediatric Surgery, Keith T. Oldham Chair in Pediatric Surgery, Children’s Wisconsin Professor of Surgery, Medical College of Wisconsin
Deb Gogin, RN, MSN, CNS
Surgical Enhancement Clinician, Children’s Wisconsin
Michael E. McCormick, MD
Complex Pediatric Otolaryngologist, Children’s Wisconsin
Professor of Pediatric Otolaryngology, Medical College of Wisconsin
David M. Gourlay, MD, FACS, FAAP
Marie Z. Uihlein Chair and Surgeon-in-Chief, Children’s Wisconsin
Professor of Pediatric General and Thoracic Surgery, Medical College of Wisconsin
Caroline Frahm, MHA
Manager of Surgical Quality and Outcomes, Children’s Wisconsin
Mary Bolhuis, RN
Surgical Clinical Enhancement Coordinator, Department of Surgical Quality, Children’s Wisconsin
Colon bundle protocol checklist
Pediatric General Surgery teams at Children’s Wisconsin use this checklist for all colorectal procedures with anastomosis and abdominal closure.
Preoperative:
• (Optional) Bowel preparation
• (Optional) Chlorhexidine (SAGE)
bath/wipes
• Umbilical cleansing (alcohol cleaning of umbilicus prior to skin prep)
• Preoperative antibiotic given within one hour of incision
– Includes gram negative and
anaerobic coverage
Intraoperative (Document in operative report):
• Anastomotic leak test
• Dedicated closure tray (instrument change
and new drapes prior to closure)
• Glove change prior to closure
• (Optional) Placement of subcutaneous drain
in grossly contaminated cases
– Drain can be: vessel loop, Penrose,
umbilical tape or other wicking object
• Maintenance of normothermia (<36 degrees
Celsius or >38 degrees Celsius for less than
30 minutes)
Postoperative:
• Perioperative antibiotics discontinued
at 24 hours
• If present, occlusive dressing removed at 48 hours to examine wound
Let’s Cope Together Helps Patients with Autism Cope with Surgery
Evidence-based approach is implemented in the perioperative setting
Children living with autism often have behavioral challenges and issues related to sensory processing and social communication. Due to comorbidities, they also are more likely to need procedural care. The nature of the perioperative environment is often bright, loud and disruptive to the patient’s routine. With the integration of evidence-based strategies, care for patients living with autism has been improved by implementing a system to develop perioperative coping plans.
Coping plans
The Diagnostic, Anesthesia and Surgical Health (DASH) team at Children’s Wisconsin has implemented the Let’s Cope Together program to develop individualized coping plans for children living with autism. During the preoperative phone call, our staff ask the family if they want to participate. If they consent, we create a personalized coping plan that may include:
• How the child best communicates
• Their likes and dislikes
• What calms them
• How they demonstrate pain
• How they take medication
We also discuss specific evidence-based environmental factors, including lighting, the number of people in the room and any safety concerns.
To facilitate the program, Children’s Wisconsin made enhancements to the electronic health record. This helps us create coping plans, track completion rates and increase awareness among care teams. Although plans are created for the day of surgery, they are also available to caregivers across our system.
We’ve seen encouraging results. In the past two years, a perioperative coping plan was in place for more than 83% of surgical patients and completion rates are trending upward. We’ve received positive feedback from staff, providers and families.
Living our values
The Let’s Cope Together program has been so successful that we also implemented it at our outpatient Surgicenter.
This initiative is a great example of collaboration at Children’s Wisconsin. It would not be successful without the teamwork of nurses, techs, child life specialists, providers and others in DASH, as well as our colleagues in Information Systems and Performance Improvement. Next steps for the project include implementing evidence-based interventions in the operating room environment.
Anita Norton, MSN, RN, CPNP-PC, CNS
Children’s Wisconsin
Lisa Boettcher, BSN, RN
Children’s Wisconsin
Jill Wiench, BS, CCLS
Children’s Wisconsin
In their words
Here are a few of the comments that parents have shared about the Let’s Cope Together program.
“I was blown away. I just couldn’t believe how well everything went.”
“This project is a great idea. I couldn’t ask for anything more.”
“It couldn’t have gone better.”
“This surgery was the best of the three that he has had.”
Announcements
Welcoming Gil Peri as Children’s Wisconsin CEO
In August, Gil Peri became President and CEO of Children’s Wisconsin.
Gil brings his expertise, values and commitment to improving the health of all children to this role. His strategic vision for Children’s Wisconsin will serve our organization and community for years to come.
Gil has held progressive health care operations and strategy leadership roles over the past 25 years. His most recent position was as president of Riley Children’s Health in Indianapolis, a 485-bed children’s health system that is part of Indiana University Health and the Indiana University School of Medicine. He has also held executive leadership roles at Connecticut Children’s Medical Center, Children’s Hospital Colorado and Nationwide Children’s Hospital.
Throughout his career, Gil has been an advocate for kids and families. This includes building key strategic partnerships among organizations with missions focused on improving the health and well-being of kids and families, such as the Riley Child Health Institute in partnership with the Indiana University School of Medicine and the Riley Children’s Care Alliance, a collaboration with other hospitals throughout Indiana. Gil also serves on the boards of the Solutions for Patient Safety and Special Olympics Indiana.
New Pediatric Urology Leader
Earlier this year, John Kryger, MD, stepped down from the role of Chief of the Division of Pediatric Urology with the Medical College of Wisconsin and Medical Director of Pediatric Urology for Children’s Wisconsin. We welcomed Douglas Storm, MD, to fill this important role.
Dr. Storm joins us from the University of Iowa Hospitals & Clinics and Stead Family Children’s Hospital, where he was a professor of Urology and Pediatrics. A U.S. Navy veteran, he completed a General Surgery internship at National Navy Medical Center in Bethesda, Maryland, followed by a Urology residency at Geisinger Medical Center in Pennsylvania and a fellowship in Pediatric Urology at Nationwide Children’s Hospital in Ohio. Dr. Storm earned his medical degree from Rush University in Chicago.
We are incredibly grateful for Dr. Kryger’s 12 years of leadership with Children’s Wisconsin and the Medical College of Wisconsin. His efforts to expand the Pediatric Urology division have grown it into one of the country’s busiest such programs. Dr. Kryger has made significant advancements in bladder exstrophy treatment and published many peer-reviewed manuscripts. Dr. Kryger will continue to see patients at Children’s Wisconsin and serve as medical director for Outreach Services in Central Wisconsin.
We thank Dr. Kryger for his years of commitment and welcome Dr. Storm to our community.
New Faculty
Keeping you informed
Connect with physician liaisons in person or virtually
The Children’s Wisconsin physician liaison team is here for referring physicians whenever and wherever you need us. In addition to serving as a link between Children’s Wisconsin and referring physicians, your liaisons can:
• Provide information about services and programs offered by Children’s Wisconsin
• Direct you to continuing education opportunities
• Facilitate solutions to referral issues
METRO MILWAUKEE
Lisa Magurany
Manager, Physician Relations
414-266-4743
lmagurany@childrenswi.org
SOUTHEAST WISCONSIN
Margie Berg
414-336-1342
mberg2@childrenswi.org
NORTHEAST WISCONSIN
Diane Dorow
920-370-9381
ddorow@childrenswi.org